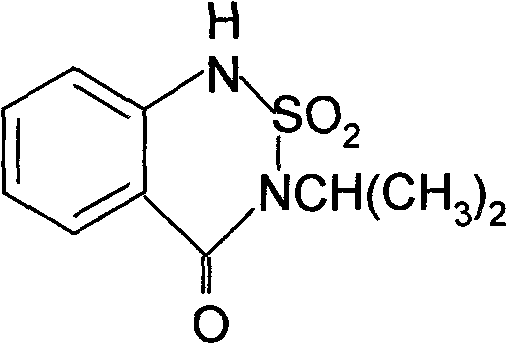
Synthetic method of bentazone
A synthetic method, the technology of bentazone, applied in the direction of organic chemistry, etc., can solve the problems of high cost of raw materials, difficult process, heavy pollution of three wastes, etc., and achieve the effect of improving the yield of cyclization, high yield and less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
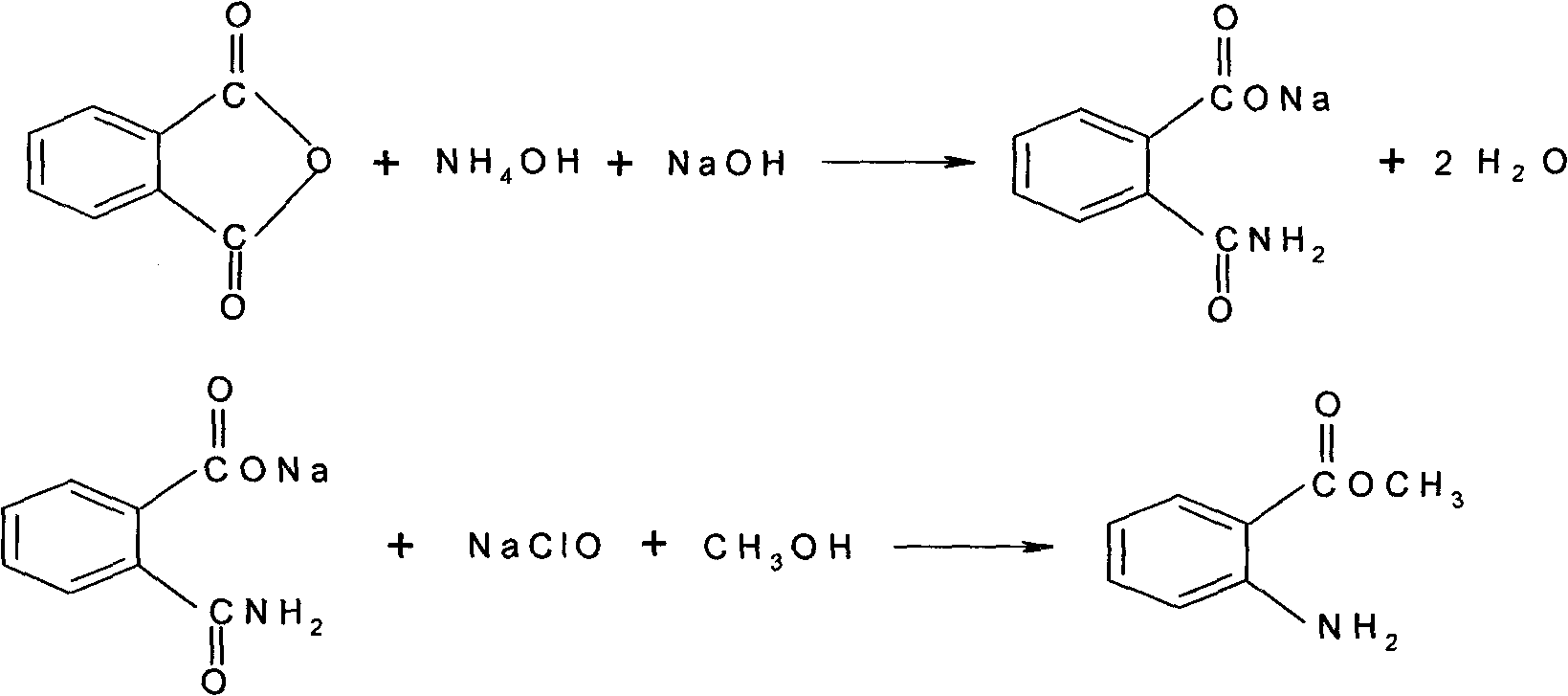
[0021] Add 6.6g (0.044M) of phthalic anhydride into the reaction flask, add 8.6g (0.044M) of 18% ammonium hydroxide solution at about 0℃, increase the temperature to 50℃, and slowly add 5.8g of 30% sodium hydroxide solution (0.044M), the speed of adding alkali should not exceed 70℃, and the reaction solution should maintain pH 8.5-8.9, so that phthalic anhydride is completely dissolved. After adding liquid alkali, adjust the pH to 12-13, 60-70 After being kept at ℃ for half an hour, the ammonia will be exhausted (about 3.5 hours) and the amide solution will be obtained. Cool to about -10℃, add 7g (0.22M) of methanol that has been pre-cooled to -10℃, stir, add pre-cooled to -10℃ 10% sodium hypochlorite solution 32.8g (0.044M). React below 0°C for 45 minutes, gradually increase the temperature to 30°C, test with potassium iodide starch test solution, it should show a colorless reaction, add appropriate amount of sodium sulfite solution (20°C) to hydrolyze, wait for the material t...
Embodiment 2
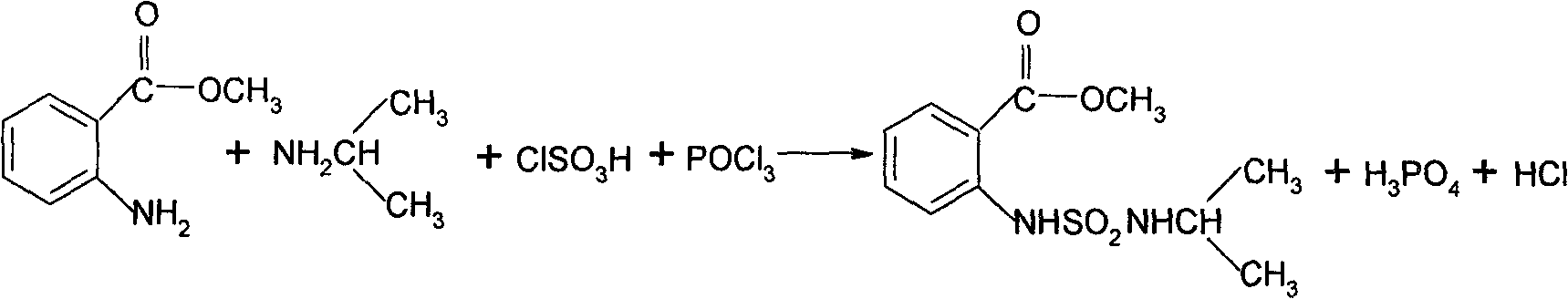
[0023] Feeding: Put 48g of dichloroethane, 17g of triethylamine (0.168M), and 6.4g (0.042M) of methyl anthranilate into reactor B. After cooling to below 10°C, start to add 5.1g of chlorosulfonic acid ( 0.044M), the temperature is controlled below 35℃, after the addition is complete, stir for 30 minutes, then add 2.6g (0.044M) of isopropylamine, stir for 30 minutes, then add 7.1g (0.046M) phosphorus oxychloride dropwise, temperature control Below 50°C, the dropwise addition is completed and stirring is completed for 30 minutes.
[0024] Pour the reacted material in the previous step into 50 g of water, stir for 30 minutes, separate the layers, add 20 mL of dichloroethane to the water layer, and extract twice again, and combine the organic layers. Add liquid caustic soda to the water layer to recover the triethylamine and apply it again.
[0025] Add the above-mentioned organic layer to the reactor C, distill it, recover the dichloroethane, and use it in a similar way. Add 22g of ...
Embodiment 3
[0027] Feeding: Put 48g of dichloroethane, 17g (0.168M) of triethylamine, 6.4g (0.042M) of methyl anthranilate into reactor B, cool to below 10°C, start adding 5.3g of chlorosulfonic acid dropwise ( 0.045M), the temperature is controlled below 35℃, after the addition is complete, stir for 30 minutes, then add isopropylamine 2.8g (0.047M), stir for 30 minutes, then add phosphorus oxychloride 7.5g (0.049M) dropwise, temperature control Below 50°C, the dropwise addition is completed and stirring is completed for 30 minutes.
[0028] Pour the reacted material in the previous step into 50 g of water, stir for 30 minutes, separate the layers, add 20 mL of dichloroethane to the water layer, and extract twice again, and combine the organic layers. Add liquid caustic soda to the water layer to recover the triethylamine and apply it again.
[0029] Add the above-mentioned organic layer to the reactor C, distill it, recover the dichloroethane, and use it in a similar way. Add 22g of methano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com