Hoofed mammal inducible multipotential stem cell and preparation method thereof
A technology of pluripotent stem cells and mammals, applied in the field of induced pluripotent stem cells and their preparation, can solve problems such as hindering the establishment of embryonic stem cell lines, and achieve the effect of improving disease resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
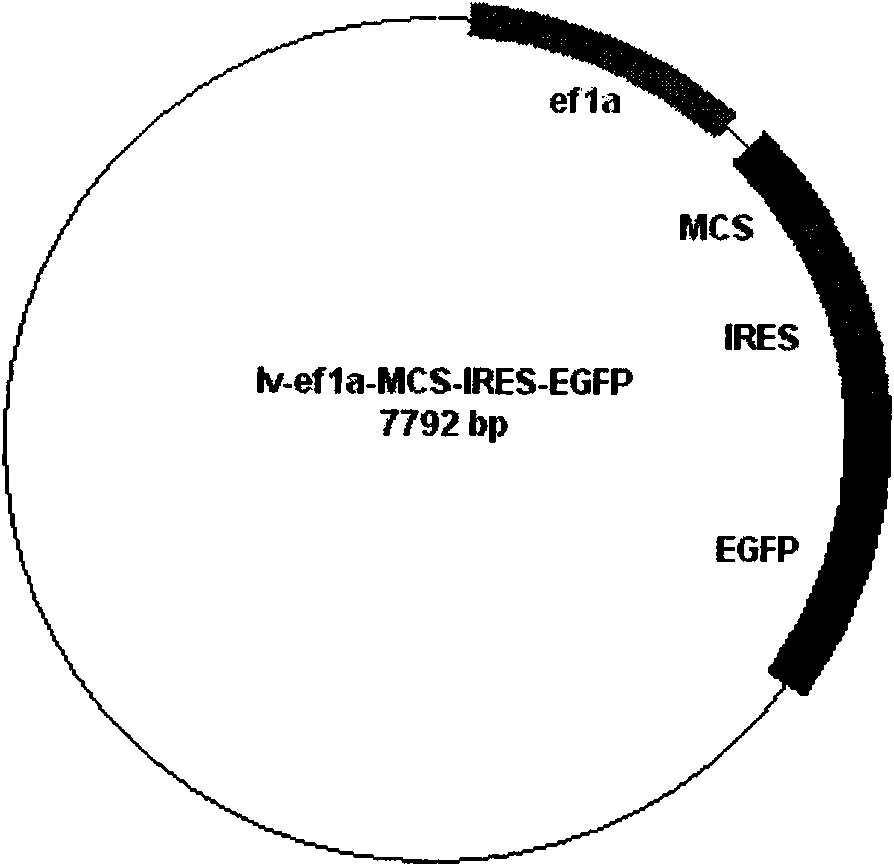
[0058] Example 1 , Construction of lentiviral vector
[0059] 1.1. Query the coding regions of specific genes (Oct4, Sox2, c-Myc, Klf4, Lin28, Nanog) specifically expressed or highly expressed in stem cells from the NCBI website (http: / / www.ncbi.nlm.nih.gov / ) , design primers according to the sequence of the coding region, and introduce restriction sites, the primer sequences are shown in Table 1 (wherein F represents the forward primer, R represents the reverse primer).
[0060] Table 1
[0061]
[0062] Note: The uppercase letters in the primer sequences are the restriction restriction sites introduced.
[0063] 1.2. PCR amplification
[0064] Using the total human cDNA as a template, PCR amplification was performed using the primers of each gene in Table 1, as follows:
[0065] Reaction system (25 μl): 2.5 μl of 10×pfx Mix, 0.2 μl of AccuPrime pfx enzyme, 0.25 μl of upstream and downstream primers (50 μM), 0.25 μl of template, and 21.55 μl of ddH2O.
[0066] Reacti...
Embodiment 2
[0070] Example 2 , cell culture
[0071] 2.1. Culture of pig and sheep primary bone marrow mesenchymal cells (BMC)
[0072] Take calf and thigh femur and tibia of newborn pig or sheep, soak them in 75% alcohol for 10 minutes, wash them with PBS containing double antibodies (penicillin, streptomycin) three times, cut the two ends of the bones, and wash with 10% fetal bovine serum (FBS) D-MEM medium sterile syringe into the bone marrow into a 50mL sterile centrifuge tube, centrifuge at 1200rpm for 5min, use the above medium to resuspend the cells once, centrifuge again at 1200rpm for 5min, resuspend the medium again and transfer the cells into in a culture bottle. Three days later, the medium was changed, and tiny clones were seen. About a week later, the cells were passaged at a ratio of 1:3. During passage, the cells were washed twice with PBS, digested with 0.25% trypsin at 37°C for 5 minutes, and then passaged at a ratio of 1:3 to 4.
[0073] 2.2. Culture of pig and shee...
Embodiment 3
[0079] Example 3 , virus packaging
[0080] 3.1. Amplification of packaging plasmid
[0081] Six kinds of correct vectors obtained in Example 1 were transformed into competent bacteria to be amplified, and Axygen AxyPrep TM After pumping in the plasma Maxiprep Kit kit (Axygen Company), purify in an ultra-clean workbench, that is, mix with 1-fold volume of 3M NaAC and 2-fold volume of absolute ethanol of the plasmid after pumping, and then centrifuge at 10,000 rpm for 15 min. Remove the supernatant, rinse with 75% ethanol, suck off the supernatant, dry it in a clean bench, use sterile deionized distilled water to dissolve the plasmid, and finally use a spectrophotometer to determine the concentration of the plasmid.
[0082] 3.2. Transfection
[0083] According to Invitrogen transfection kit (ViraPower TM Lentiviral Expression Systems), using the transfection reagent Lipofectamine TM In 2000, the six lentiviral plasmids in step 3.1 were combined with the packaging plas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com