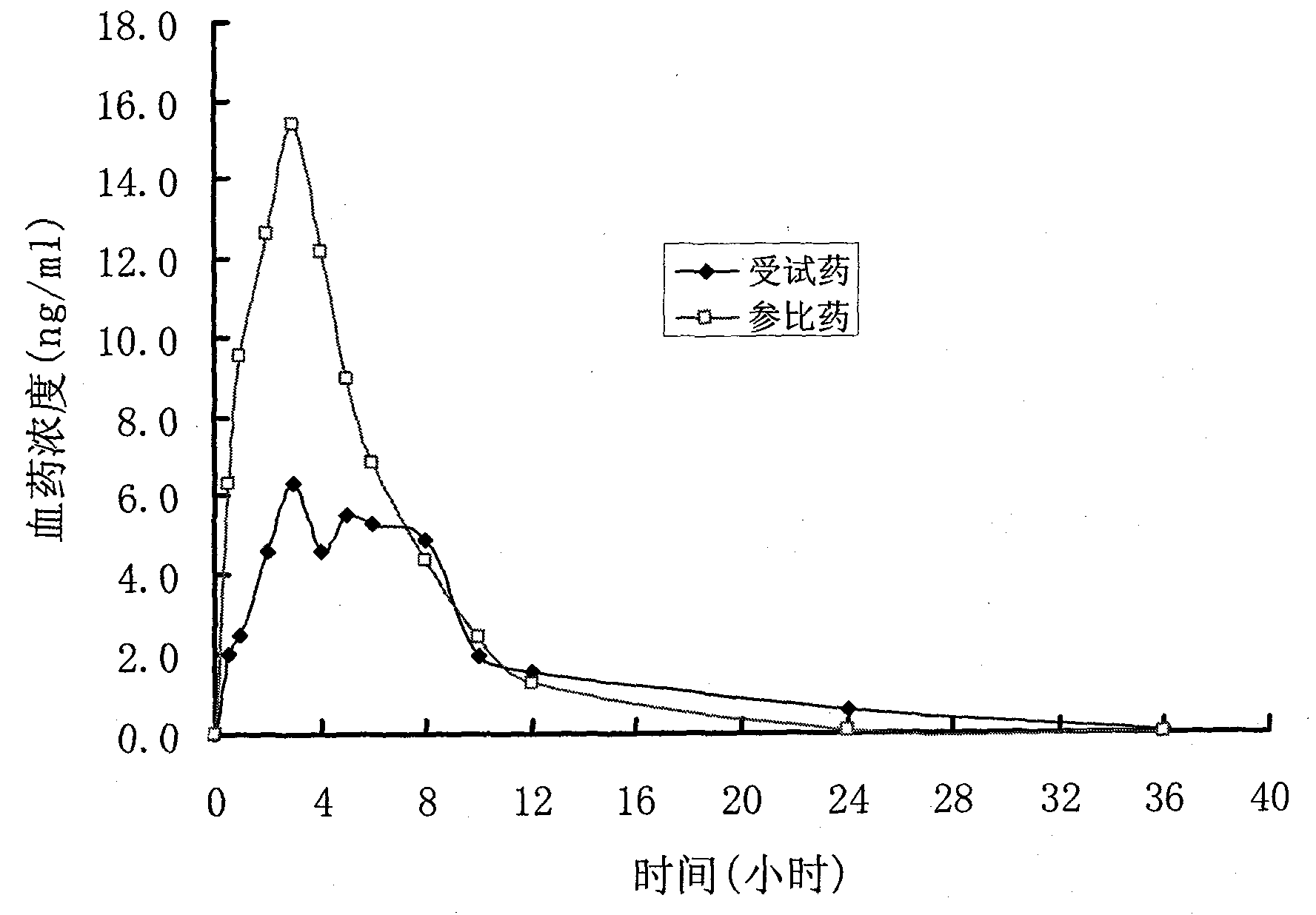
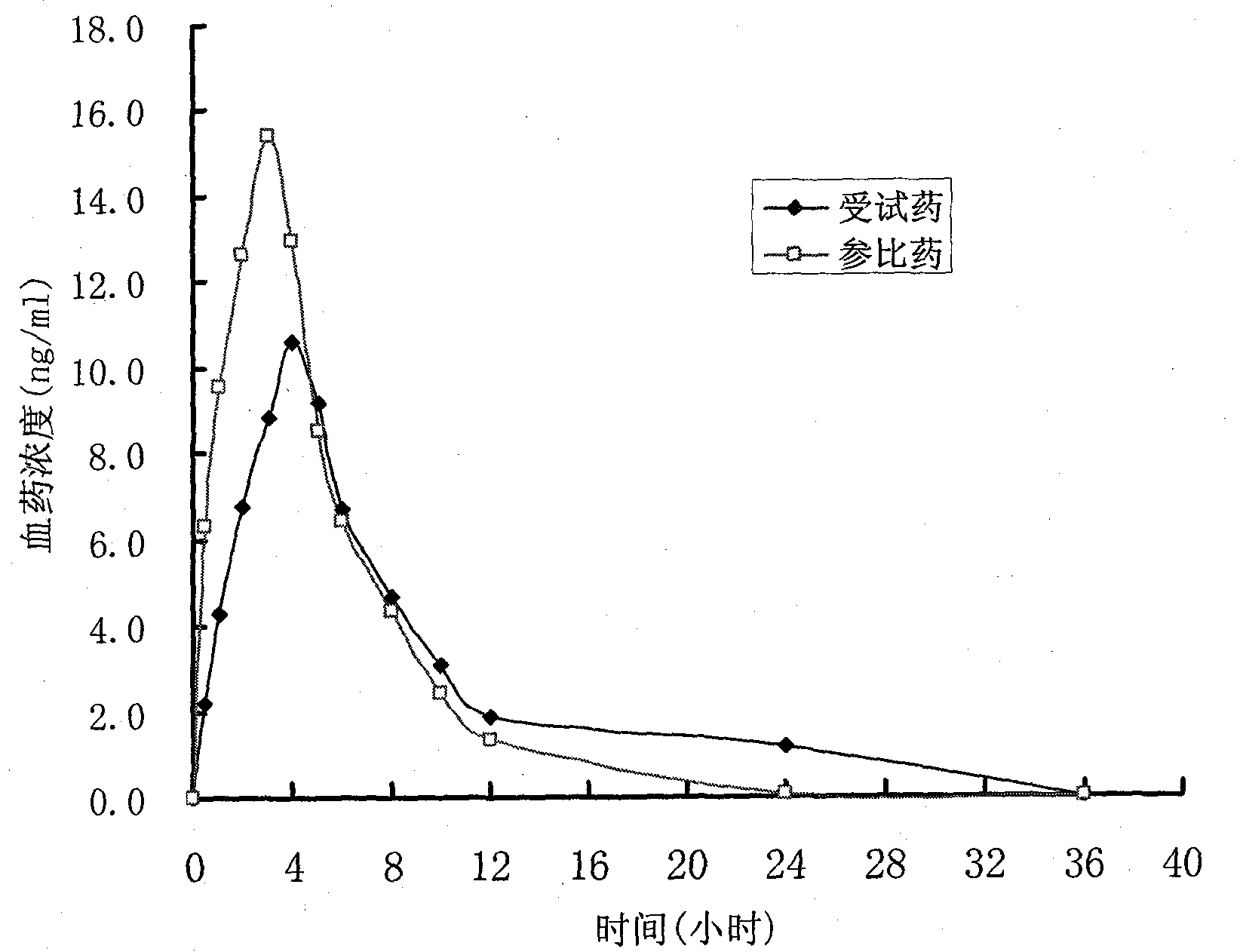
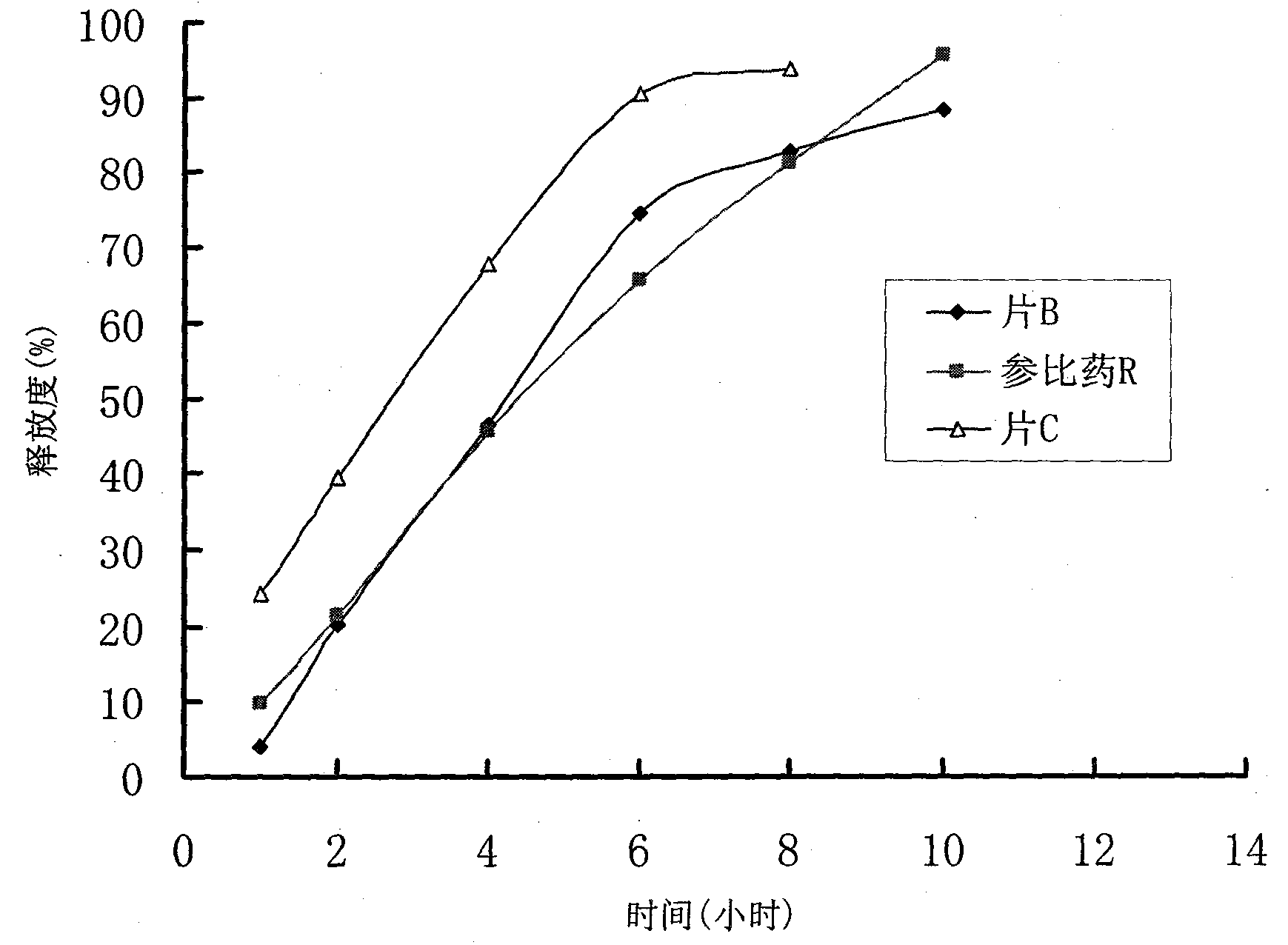
Controlled release preparation and preparation method thereof
A technology for controlled release preparations and preparations, which is applied in the field of pharmacy and can solve the problems of incomplete release and delay of active ingredients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Prepare tablet cores according to the following prescription
[0034]
[0035] (1) According to the above drug layer prescription, under a dry environment, pass felodipine through a 100-mesh sieve and fully mix it with lactose, sodium chloride, and polyoxyethylene, and use a dry granulator to make granules with a 20-mesh sieve . Add magnesium stearate and mix well to form drug layer granules.
[0036] (2) According to the above-mentioned propulsion layer prescription (excluding magnesium stearate), after mixing uniformly by conventional methods in a dry environment, use a dry granulator to make granules with an 18-mesh screen. Add magnesium stearate and mix evenly to become push layer granules.
[0037] (3) Place the drug layer granules and the push layer granules respectively in the hopper of a double-layer tablet press, adopt a circular shallow arc die with a diameter of 8mm, and adjust the weights of the drug layer and the push layer to be close to the above-men...
Embodiment 2
[0054] Prepare tablet cores according to the following prescription
[0055]
[0056] (1) Prepared with (1)~(5) of Example 1, the difference is that the amount of felodipine contained in the drug layer in the double-layer tablet core prepared this time is about 4 mg.
[0057] (2) Opadry is placed in 70% ethanol solution, so that the content of Opadry in the solution is about 8%, fully stir with a stirrer to dissolve the hypromellose in it, then add felodipine to make the package The content of felodipine in the coating solution is about 0.3%, and the felodipine is fully stirred with a stirrer or a shear emulsifier to dissolve or fully suspend and maintain stirring. The double-layer tablet prepared above is placed in a coating machine, the air inlet temperature is adjusted to about 45° C., and the coating solution is sprayed on the double-layer tablet until the coating layer contains about 1 mg of felodipine. The drug layer of the double-layer tablet of this product made in t...
Embodiment 3
[0069] Prepare tablet cores according to the following prescription
[0070]
[0071] (1) Same as steps (1) to (3) in Example 1, to make a tablet core with a diameter of 9 mm.
[0072] (2) cellulose acetate and polyethylene glycol 400 (consumption=18% cellulose acetate weight) are dissolved in dichloromethane, make the total solid content containing cellulose acetate and PEG 400 in the dichloromethane be about 5% , stir to dissolve it completely and become a semi-permeable membrane solution. The tablet core is placed in a coating machine, the air inlet temperature is adjusted to 35-45° C., the semipermeable membrane solution is sprayed on the tablet core until the weight gain of the tablet core is about 14%, and a double-layer tablet is obtained.
[0073] (3) Use a laser puncher to punch a hole with a diameter of about 0.4-0.7 mm in the center of the side film of the drug layer of each sheet. The received piece is called piece D.
[0074] (4) The stomach-soluble film coa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com