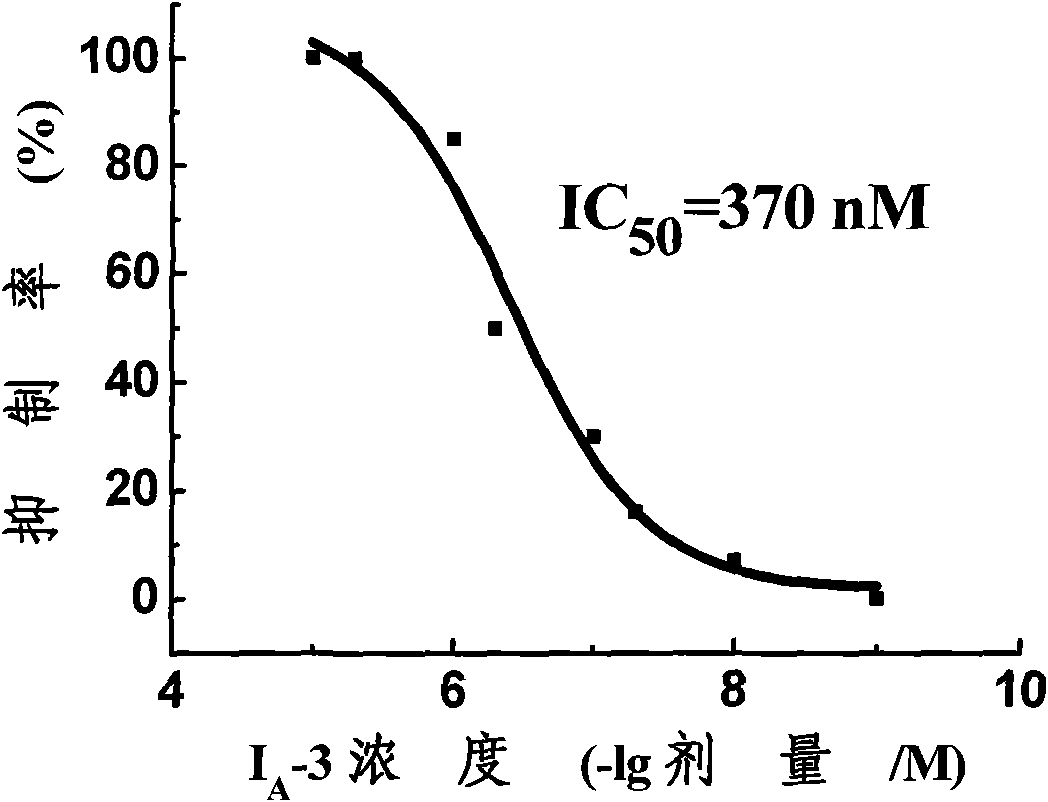
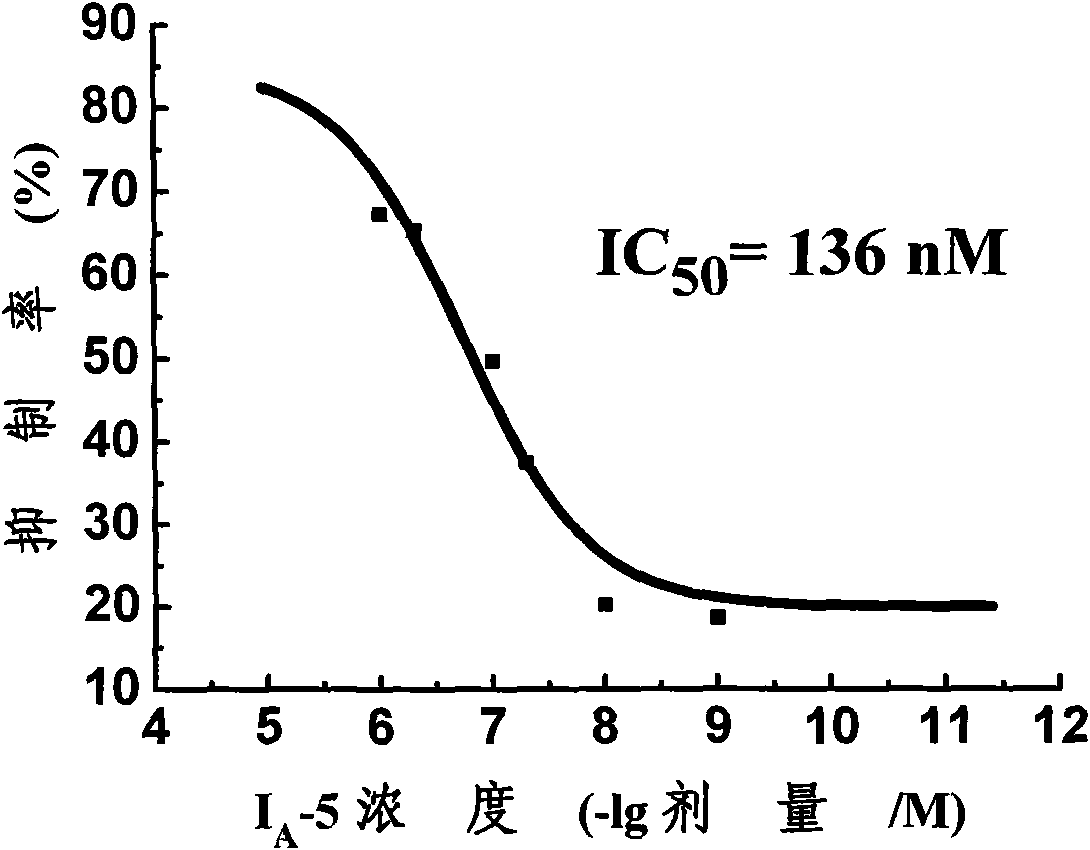
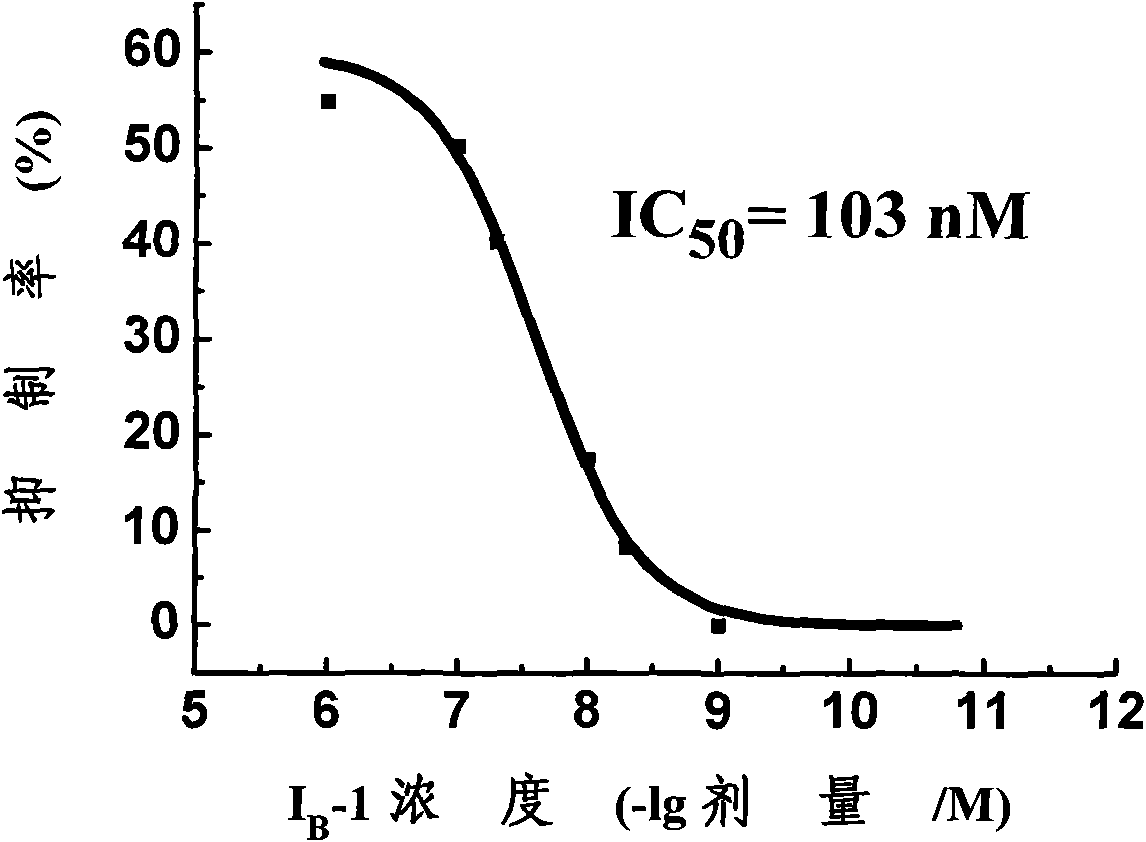
Substituted benzoyl urea compound and preparation method and application thereof
A technology of benzoylureas and compounds, applied in the field of substituted benzoylureas compounds and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0077] Example 12, 6-dibenzyloxybenzonitrile (intermediate VIII)
[0078]
[0079] Dissolve 0.5 g of sodium benzyl alcohol in 10 ml of dimethyl sulfoxide, and add 2,6-difluorobenzonitrile under stirring. After rapidly raising the temperature to 115°C, stirring was continued for 8 hours. After cooling to room temperature, 100 ml of ice water was poured into the reaction liquid, and stirred for 30 minutes. A large amount of solid precipitated, which was suction filtered, washed with water, and dried to obtain a white solid. Recrystallize from ethanol to obtain intermediate VIII in the form of white flaky crystals. 1 H-NMR (400Hz, DMSO-d 6 )δ: 5.19 (4H, s, -CH 2 ), 6.60 (2H, d, Ar-H), 7.30-7.35 (3H, m, Ar-H), 7.38 (4H, t), 7.45 (4H, d).
Embodiment 2
[0080] Example 2 2,6-dibenzyloxybenzamide (intermediate IX)
[0081]
[0082] Dissolve 0.4 g of 2,6-dibenzylbenzonitrile (VIII) in 7 ml of benzyl alcohol, add 0.6 g of KOH and 0.1 ml of water, and stir at 130° C. for 12 hours. The solvent was distilled off under reduced pressure, and 100 ml of ice water was poured into the reaction liquid, stirred for 30 minutes, a large amount of solid precipitated out, filtered with suction, washed with water, and dried to obtain a white solid. Silica gel column chromatography (petroleum ether / ethyl acetate=12 / 1) gave intermediate IX as a white powdery solid. 1 H-NMR (400Hz, DMSO-d 6 )δ: 5.15 (4H, s, -CH 2 ), 6.60 (2H, d, Ar-H), 7.18-7.48 (12H, m, Ar-H); EI-MS m / z 333.2 (M + ), 91.1 (100%).
Embodiment 3
[0083] Example 3 1-(1-xanthenyl)-3-(2,6-dibenzyloxybenzoyl)urea (intermediate XI-1)
[0084]
[0085] Dissolve 0.5 g of triphosgene in 3 ml of 1,2-dichloroethane, and stir in an ice bath until dissolved. 0.25 g of xanthamine was dissolved in 10 ml of 1,2-dichloroethane, and slowly added dropwise into the reaction solution containing triphosgene, the reaction solution was white and turbid. After continuing the ice bath for 0.5 hours, the temperature was raised to 25° C. for 1 hour, and then heated under reflux until the reaction solution was colorless and transparent, and 1 ml of triethylamine was added to the reaction solution to continue reflux for 0.5 hours. The solvent was distilled off under reduced pressure, 10 ml of toluene was added, and suction filtered. The filtrate was not post-treated, and 0.25 g of 2,6-dibenzyloxybenzamide (IX) was added thereto, and refluxed for 10 hours. The solvent was evaporated under reduced pressure, and water was added to precipitate a s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com