Ruthenium-containing coordination compound and preparation method thereof
A complex and inhibitor technology, applied in the field of ruthenium-containing complexes and their preparation, can solve the problems of no "single-molecule multi-target" anti-tumor drug research reports, stay in laboratory research, toxic and side effects, etc. The effect of reducing the possibility of drug resistance, improving water solubility, and reducing toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] According to the present invention, the preparation method of the complex containing ruthenium comprises the following steps:
[0019] (1) In the mixed solution of liquefied ammonia and lower alcohol, aromatic hydrocarbons are mixed with alkali metals to obtain dihydroaromatic hydrocarbons;
[0020] (2) contacting the dihydroaromatic hydrocarbon with the ruthenium halide in the first organic solvent, and the conditions of the contact reaction make the reaction obtain the ruthenium halide arene dimer;
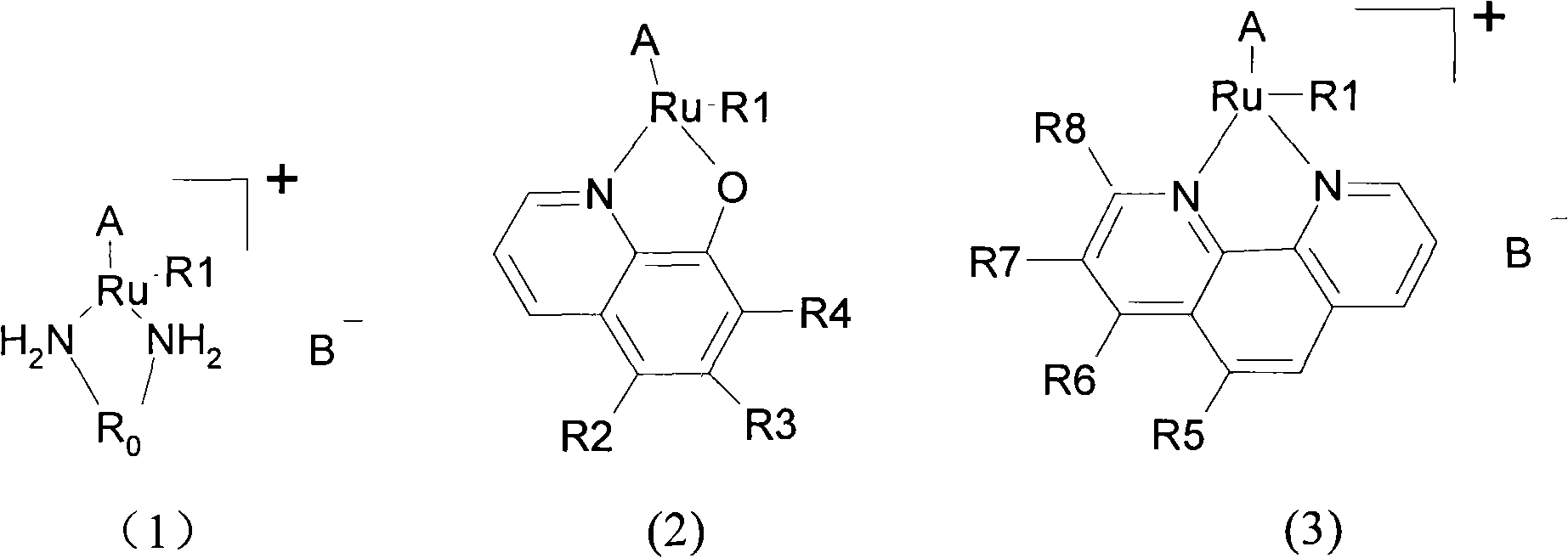
[0021] (3) The ruthenium halide arene dimer that step (2) obtains is contacted with the chelating ligand that contains two coordination atoms in the second organic solvent, and the chelating ligand that contains two coordination atoms is selected from From one of alkyldiamine, 8-hydroxyquinoline and its derivatives, and phenanthroline and its derivatives, the conditions of the contact reaction make the ruthenium in the ruthenium halide arene dimer and the two in the chela...
Embodiment 1
[0035] This example serves to illustrate the preparation of the ruthenium-containing complexes of the present invention.
[0036]
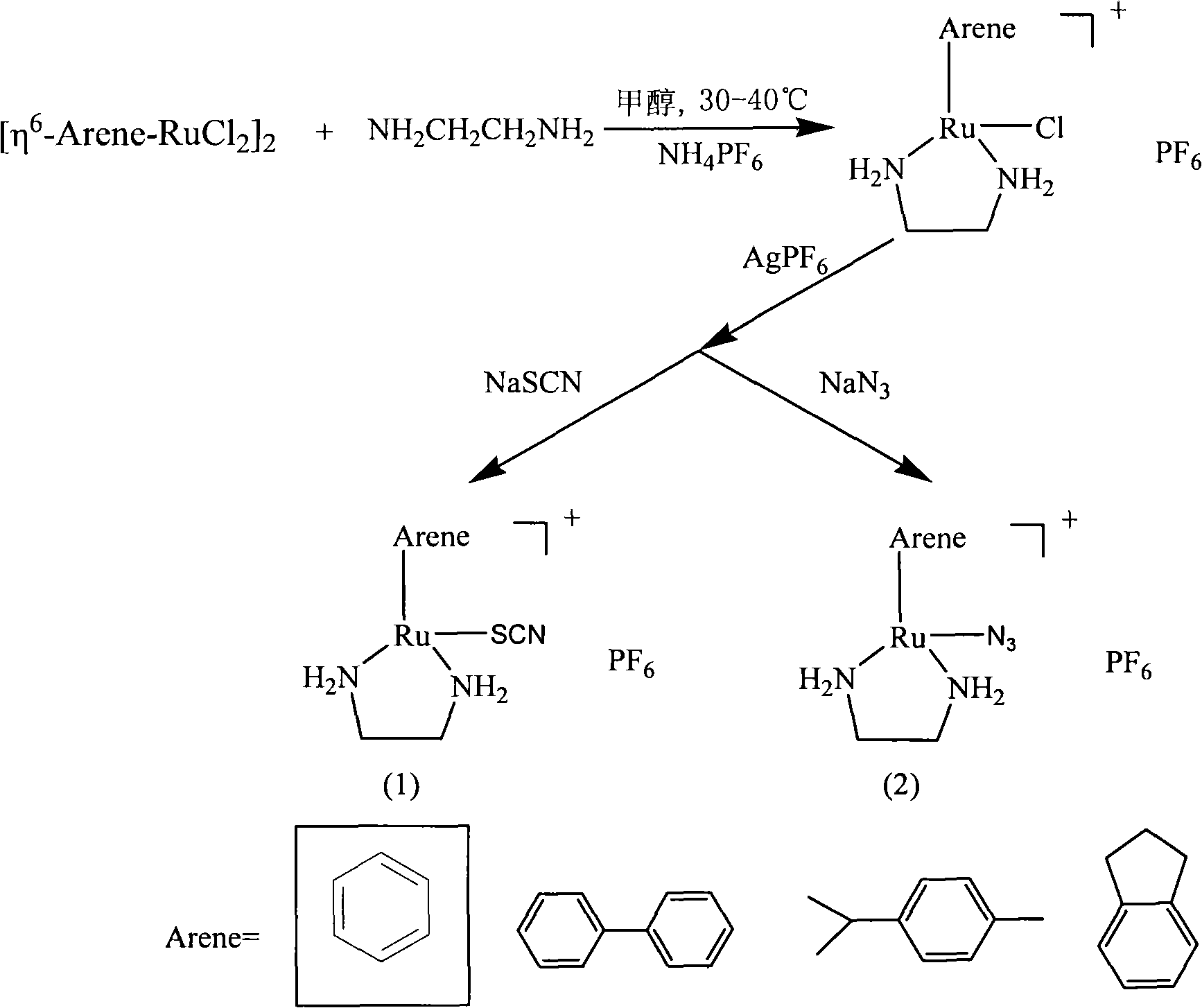
[0037] (1) In the mixed solution of liquid ammonia and ethanol, at -78°C, mix the aromatic hydrocarbon and metallic sodium for 1 hour (the molar ratio of liquid ammonia, ethanol, aromatic hydrocarbon and sodium is 250:10:1:5), to obtain Dihydrogenated aromatic hydrocarbons; the aromatic hydrocarbons are respectively benzene, biphenyl, isopropyl-p-toluene, and benzocyclopentane; then the reaction product is subjected to vacuum distillation at 50-150° C. to remove the solvent and some unreacted raw materials, Obtained by nuclear magnetic resonance analysis, in the reaction product mixture, the purity of dihydroaromatics is about 70% by weight;
[0038] (2) The reaction product mixture containing dihydroaromatics and ruthenium chloride are contacted in ethanol, wherein, the consumption of the reaction product mixture containing dihydroaromatics ma...
Embodiment 2
[0055] This example serves to illustrate the preparation of the ruthenium-containing complexes of the present invention.
[0056]
[0057] The ruthenium chloride arene dimer (prepared in Example 1) with a molar ratio of 1:1 was contacted with 8-hydroxyquinoline and its derivatives at 35° C. in methanol solution for 10 hours, and the ruthenium chloride The total amount of arene dimer and 8-hydroxyquinoline and its derivatives is 100 mg as a basis, and the amount of methanol is 30 ml, and then recrystallized in ethanol solution.
[0058] The benzene-ruthenium series complexes were obtained respectively: LQ2009, LQ2010, LQ2011 and LQ2012;
[0059] p-cymene-ruthenium complexes: LQ2013, LQ2014, LQ2015 and LQ2016.
[0060] The compounds prepared above were characterized by nuclear magnetic resonance and mass spectrometry respectively:
[0061] LQ2009: C 15 h 12 NORuCl, calc.MW=358.8, ESI-MS (m / z): [M-Cl] + =323.3
[0062] 1 H NMR: (Deuterated DMSO) δ (ppm): 9.315 (d, 1H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com