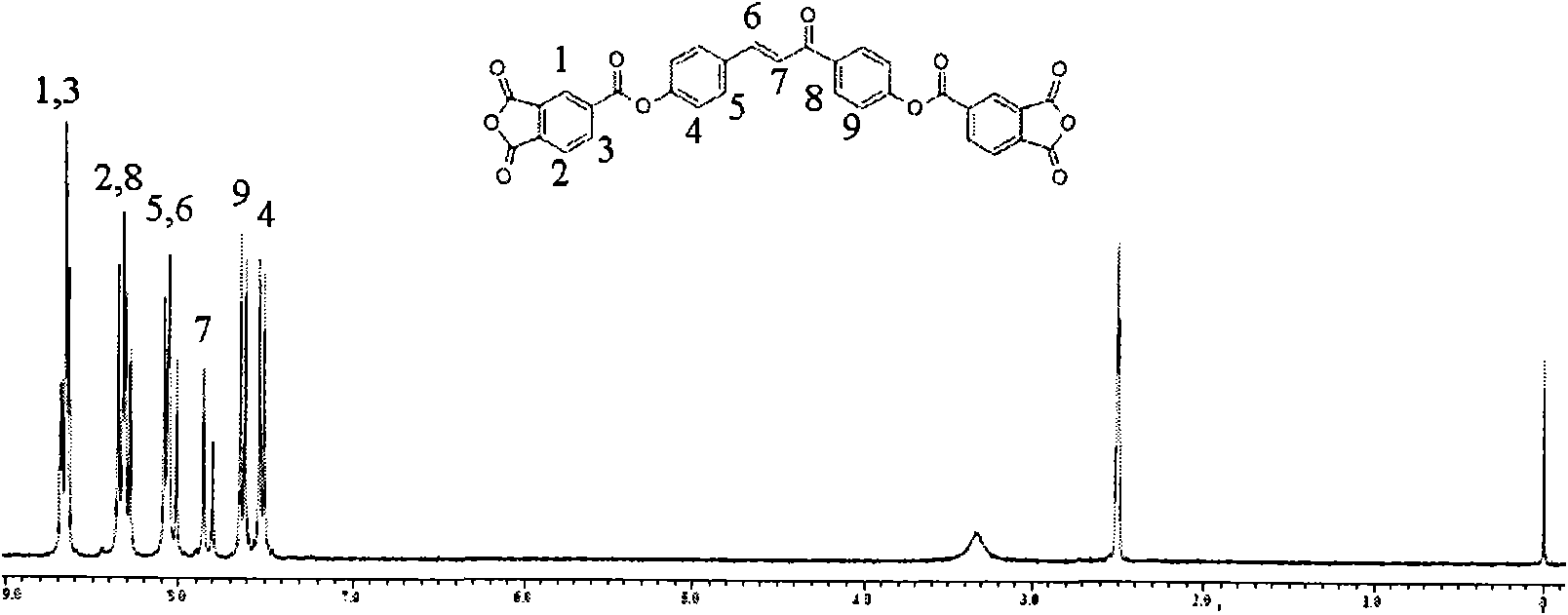
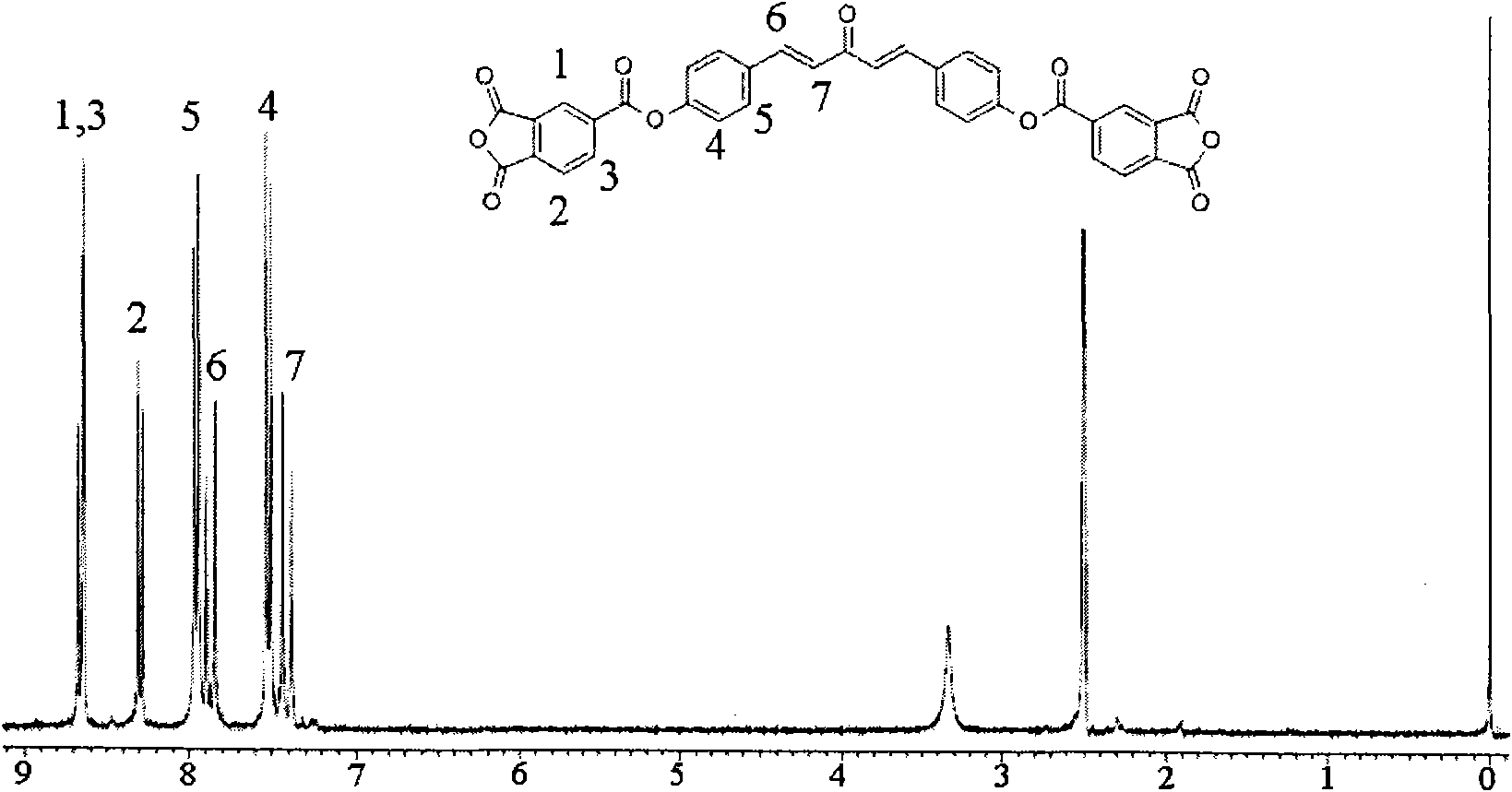
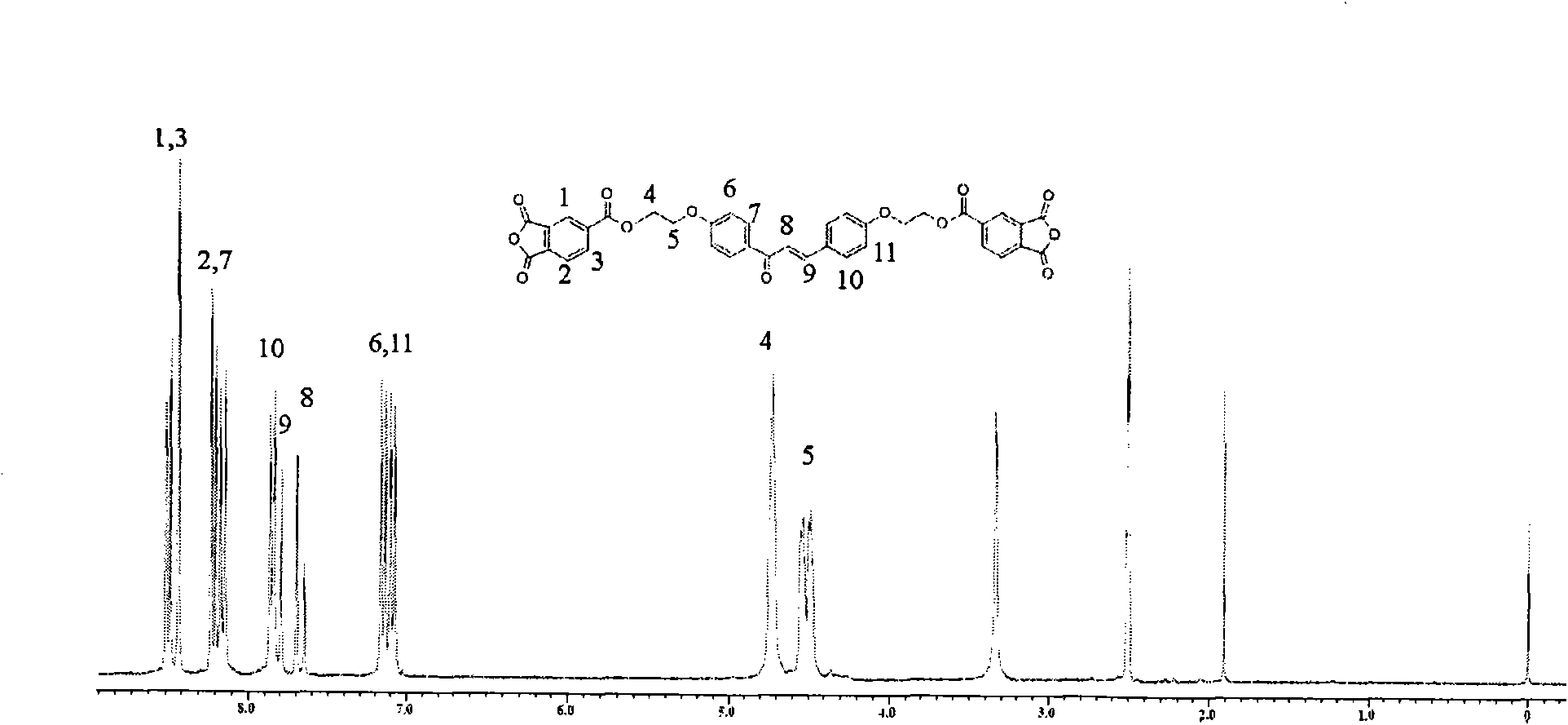
Dianhydride monomer containing photocrosslinkable phenyl vinyl ketone element and preparation method thereof
A technology for cross-linking phenyl vinyl ketone and phenyl vinyl ketone, applied in the direction of organic chemistry, etc., can solve problems such as complex synthesis, and achieve the effects of high purity, simple control of synthesis process conditions, and simple raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] A preparation method for a dianhydride monomer containing photocrosslinkable phenyl vinyl ketone units, comprising the following steps:
[0046](2-1) Condensation reaction of aromatic aldehyde and ketone to synthesize dihydroxyphenyl vinyl ketone with two phenolic hydroxyl groups: the molar ratio of aromatic aldehyde to ketone is: aromatic aldehyde: ketone = 1 ~ 1.1: 1 or 2 ~2.2:1, the reaction temperature is 0~10℃, the reaction time is 8~16 hours, then add dilute hydrochloric acid to neutralize to pH=5~7, produce precipitate, filter, wash, and dry at 70~100℃ for 6~15 hours Hour, recrystallization, obtain the dihydroxyphenyl vinyl ketone with two phenolic hydroxyl groups;
[0047] (2-2) Synthesis of dihydroxyalkylphenyl vinyl ketone ether with two alcoholic hydroxyl groups: the above-mentioned dihydroxyphenyl vinyl ketone with two phenolic hydroxyl groups is dissolved in a solvent, and the solvent is dimethyl sulfoxide Or N, N-dimethylformamide, made into a homogeneous...
Embodiment 1
[0052] (1-1) Synthetic phenyl vinyl ketone with two phenolic hydroxyl groups:
[0053] Weigh 0.11mol of p-hydroxybenzaldehyde and 0.1mol of p-hydroxyacetophenone into a 250mL single-mouth bottle, add 50mL of ethanol to dissolve, and place in an ice-water bath; weigh 33g of NaOH solid, slowly dissolve it in 33mL of water, and make a concentration It is a 50% NaOH solution, cooled to about 0°C; under constant stirring, slowly add the concentrated alkali solution dropwise to the ethanol solution of aldehydes and ketones. After the dropwise addition, continue to react at below 10°C for 12 hours, neutralize with 1mol / L dilute hydrochloric acid until the pH is around 5, filter, wash with water, and dry at 75°C for 8 hours, then recrystallize with water to obtain a light yellow filament Solid, yield 50%.
[0054] (1-2) synthesis of 1,2,4-benzenetriacid anhydride acid chloride:
[0055] Weigh 38.4g of trimellitic anhydride into a 100mL single-necked bottle, add 50mL of thionyl chlor...
Embodiment 2
[0060] (2-1) Synthesis of phenyl vinyl ketone with two phenolic hydroxyl groups: similar to the step (1-1) of Example 1, except that p-hydroxybenzaldehyde was replaced by m-hydroxybenzaldehyde, the yield was 68%.
[0061] (2-2). Synthesis of 1,2,4-trimellitic anhydride acid chloride: the same as the step (1-2) of Example 1.
[0062] (2-3) Synthesis of a dianhydride monomer containing phenyl vinyl ketone: similar to the step (1-3) of Example 1, except that the 4,4'-dihydroxyl compound synthesized in the step (1-1) is Alone is replaced by the product synthesized in step (2-1), yield 60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com