Application of ligustilide in preparing composition for preventing and treating senile dementia
A technology of ligustilide and senile dementia, which is applied in the field of application of ligustilide in the preparation of compositions for preventing and treating senile dementia, and can solve the problems of affecting the clinical prevention and treatment effect of senile dementia, poor long-term treatment effect, large adverse reactions, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
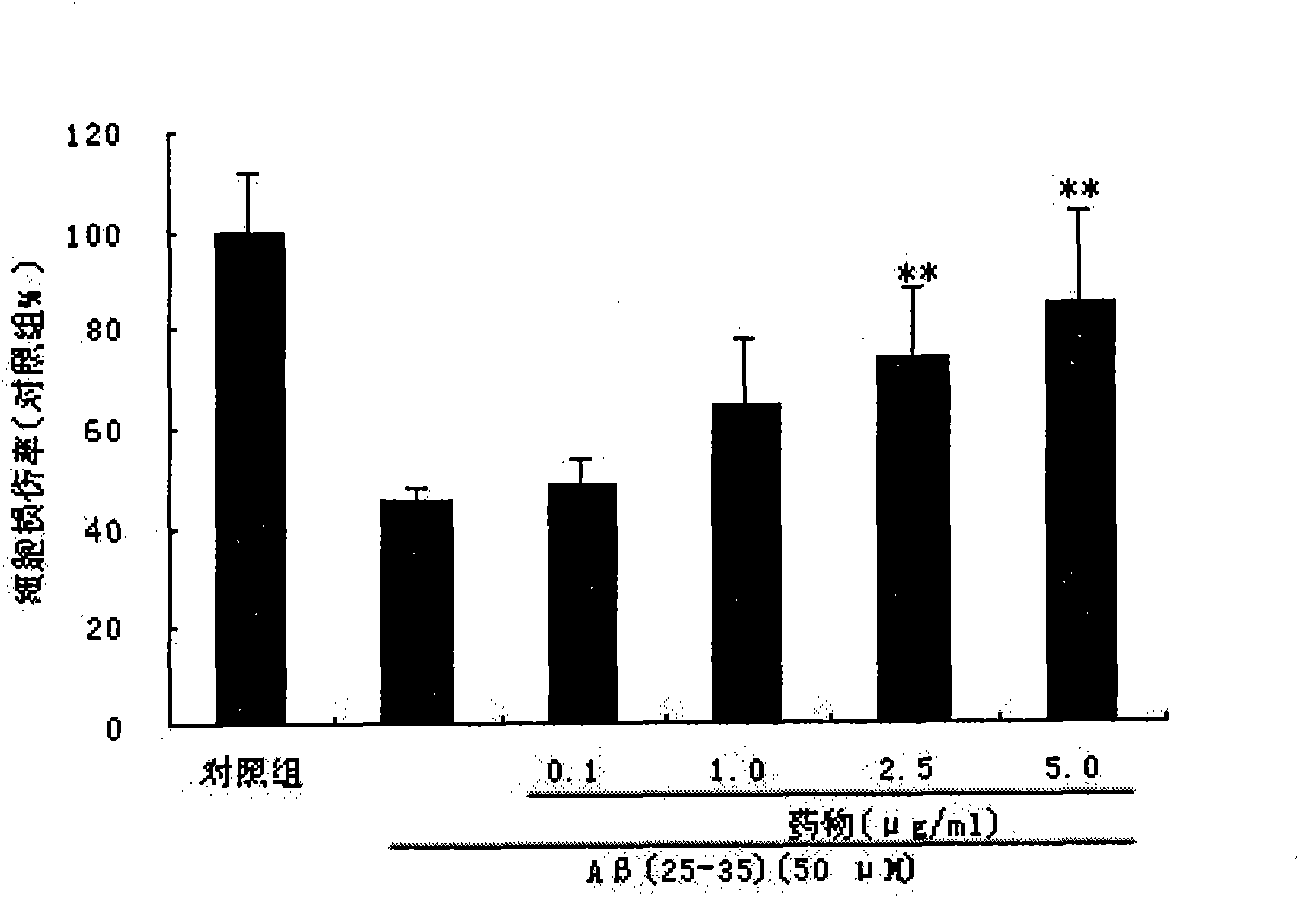
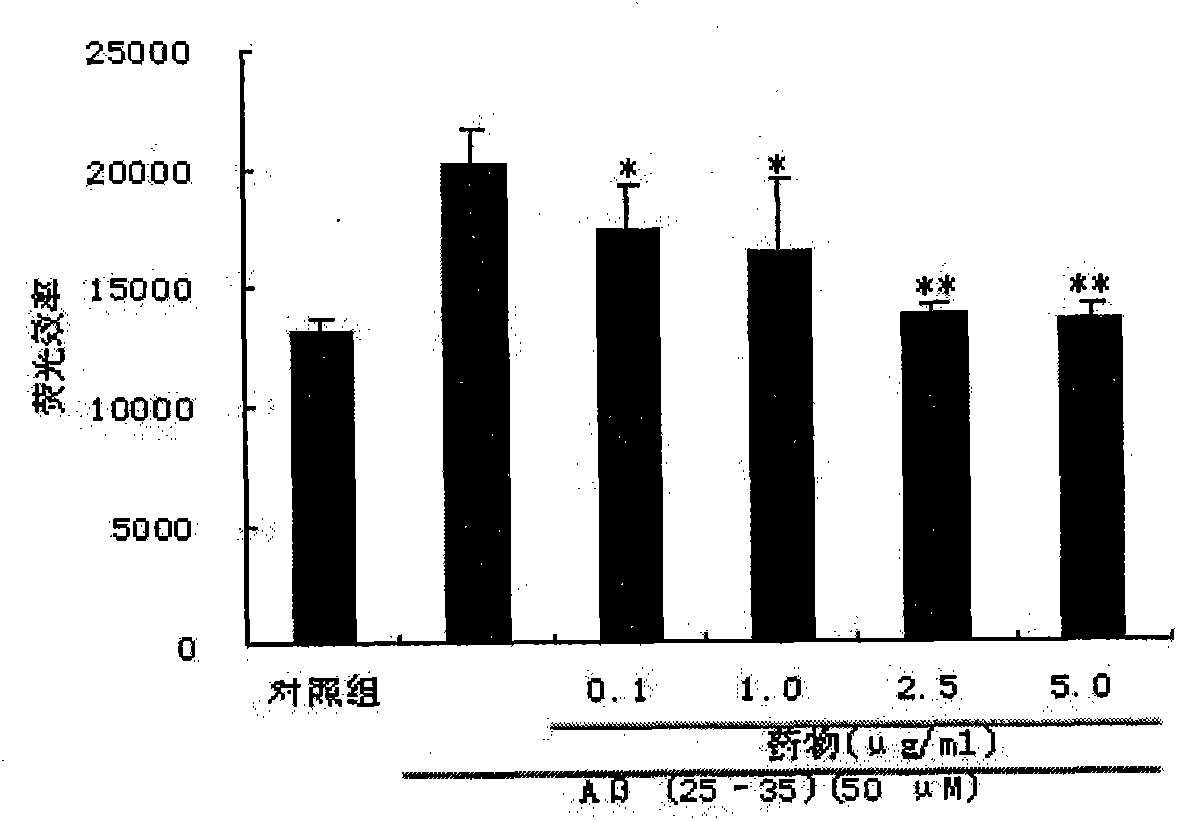
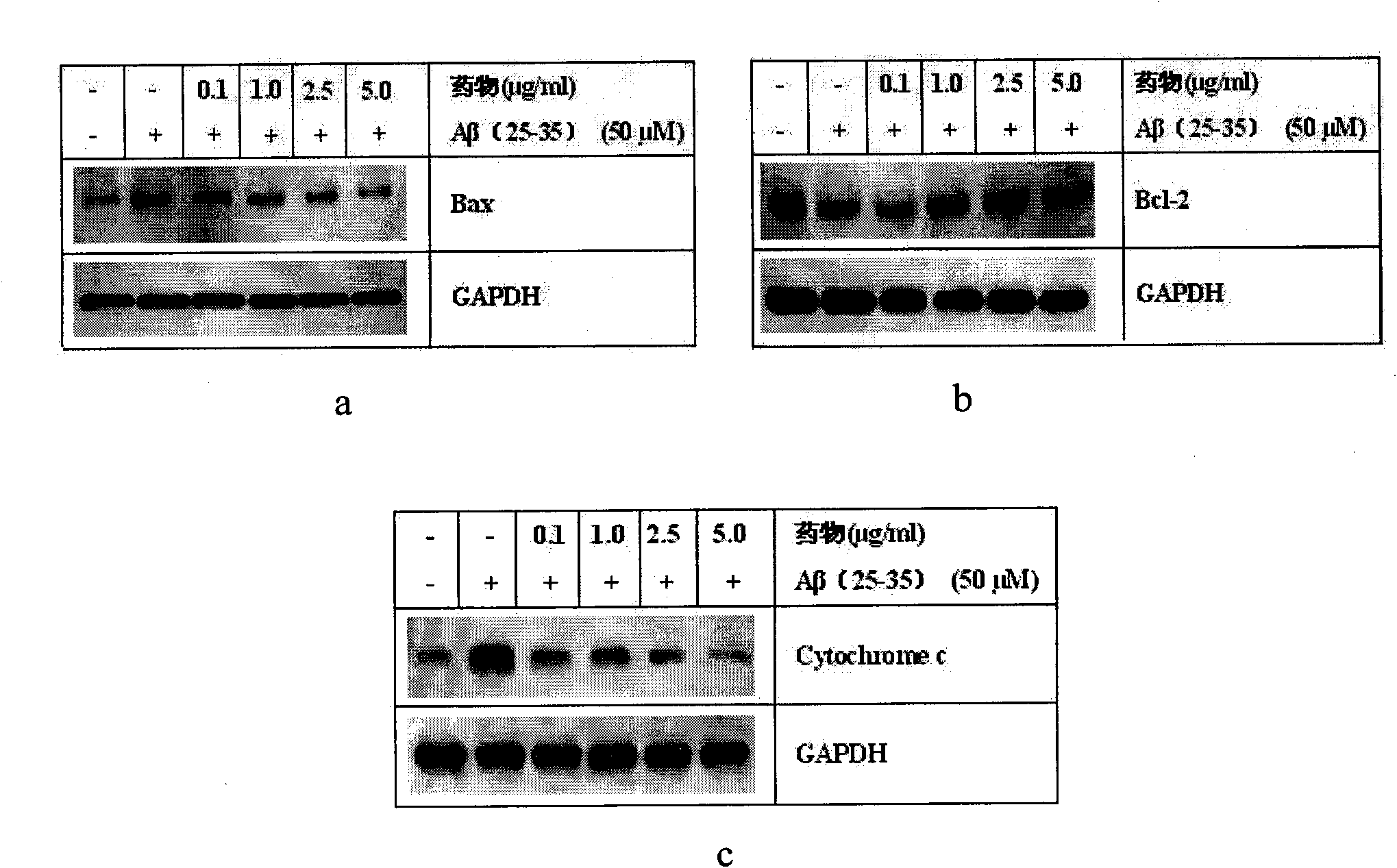
[0034] Ligustilide (LIG) protects SH-SY5Y cell injury induced by Aβ amyloid peptide
[0035] Cell culture: human neuroblastoma cells SH-SY5Y cell line was purchased from ATCC, USA. SH-SY5Y cells use high-glucose DMEM complete medium [containing 10% (V / V) FBS, 1% (V / V) NEAA, 100U / ml penicillin, 100μg / ml streptomycin], at 37°C, 5% CO 2 , cultured in a cell incubator with saturated humidity. The culture medium was changed every 2-3 days and subcultured every 4-6 days. Cells in the logarithmic growth phase were inoculated on culture plates for different experiments.
[0036] Reagents and medicines:
[0037] N-2supplement American Gibco company
[0038] Non-Essential Amino Acids (NEAA) Sigma Corporation of America
[0039] Aβ(25-35) Sigma, USA
[0040] TNF-α polyclonal antibody American CST company
[0041] Cox2 polyclonal antibody US CST company
[0042] The rest of the reagents were commercially available analytically pure.
[0043] instrument:
[0044] ESCO Class II B...
Embodiment 2
[0069] Effects of Ligustilide on Memory Impairment in Rats Induced by Brain Stereotaxic Injection of Aβ Amyloid Peptide
[0070] Animals: male Wistar rats, weighing 300-350 g, SPF grade, purchased from the Institute of Experimental Animals, Academy of Sciences, Sichuan Medical Academy. After the room temperature was kept at about 25°C, they were free to eat and drink.
[0071] Reagents and medicines: the chemical purity of ligustilide used is >98%, prepared with 3% Tween-80;
[0072] The rest of the reagents were commercially available analytically pure.
[0073] Instruments: Morris water maze, recorder, etc.
[0074] Preparation of Aβ(25-35) solution:
[0075] Aβ(25-35) solution (5mM / l) was prepared with sterile physiological saline, aged at 37°C for 7 days according to the literature method, and then used for later use.
[0076] Model establishment of memory dysfunction in rats induced by stereotaxic injection of Aβ amyloid peptide:
[0077] After the experimental rats ...
Embodiment 3
[0086] Effects of ligustilide on neuronal injury in rats induced by stereotaxic injection of Aβ amyloid peptide
[0087] Animals: male Wistar rats, weighing 300-350 g, SPF grade, purchased from the Institute of Experimental Animals, Sichuan Academy of Medical Sciences. After the room temperature was kept at about 25°C, they were free to eat and drink.
[0088] Reagents and medicines: the chemical purity of ligustilide used is >98%, prepared with 3% Tween-80;
[0089] The rest of the reagents were commercially available analytically pure.
[0090] Preparation of Aβ(25-35) solution
[0091] Prepare Aβ(25-35) solution (5mM / l) with sterile normal saline, and aging it at 37°C for 7 days according to the method in the literature.
[0092] Establishment of a model of memory dysfunction in rats induced by stereotaxic injection of Aβ amyloid peptide
[0093] After the experimental rats were anesthetized with 1% sodium pentobarbital (40 mg / kg), the head was fixed with a stereotaxic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com