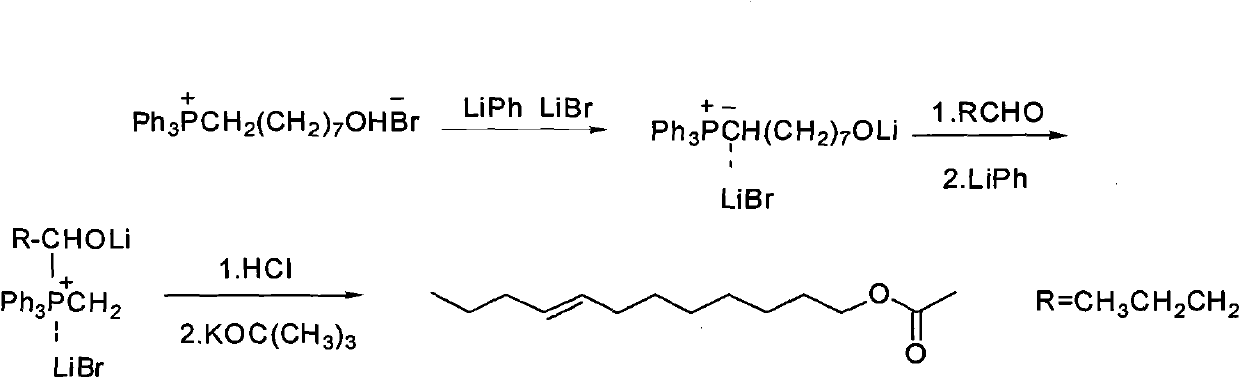
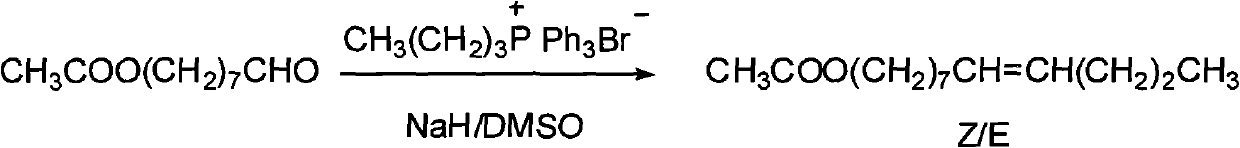Synthesizing method of oriental fruit moth sex pheromone 8(Z/E)-dodecylene-1-alcohol acetate
A technology of pear borer pheromone and dodecene, which is applied in the field of chemical synthesis of insect sex pheromones, can solve the problems of difficult acquisition of raw materials and solvents, easy residue, harsh reaction conditions, etc., and achieve high activity of attracting small borer worms , High product yield and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Under nitrogen protection, 5kg (10.64moL) 8-hydroxyoctyltriphenylphosphine salt dissolved in 7.5L organic solvent (2.5L DMF and 5L toluene) was cooled to 0°C in the reactor, and 415g (10.64moL )NaNH 2 Add to the reaction kettle in batches, and continue to react for 30 minutes after the dropwise addition to make it fully react; take 510g (12.75moL) NaH (content 60%) and add it to the reaction kettle in batches, stir until the system is dark red, slowly add 770g (10.68moL) ) with the n-butyraldehyde diluted with 7.5L organic solvent (2.5L DMF and 5L toluene), the dropwise addition is completed, and the reaction is continued until the crimson color fades away, and the reaction ends. Pour the reaction solution into ice water to inactivate, adjust the pH to neutral with hydrochloric acid, extract with hexane, dry, concentrate the organic phase, and distill the crude product under reduced pressure to obtain the target product 8(Z / E)-dodecen-1-ol 1.8kg, this step yield is 92%...
Embodiment 2
[0036] Under nitrogen protection, 5kg (10.64moL) 8-hydroxyoctyltriphenylphosphine salt dissolved in 10L organic solvent (2.5L DMF and 7.5L benzene) was cooled to 0°C in the reactor, and 415g (10.64moL )NaNH 2Add to the reaction kettle in batches, and continue to react for 60 minutes after the dropwise addition to make it fully react; take 640g (16moL) NaH (content 60%) and add it to the reaction kettle in batches, stir until the system appears dark red, slowly add 840g (11.65moL) With n-butyraldehyde diluted with 10L organic solvent (2.5L DMF and 7.5L toluene), the dropwise addition is completed, and the reaction is continued until the crimson color fades, and the reaction ends. Pour the reaction solution into ice water to inactivate, adjust the pH to neutral with hydrochloric acid, extract with hexane, dry, concentrate the organic phase, and distill the crude product under reduced pressure to obtain the target product 8(Z / E)-dodecen-1-ol 1.82kg, this step yield is 93%. Acco...
Embodiment 3
[0039] Under the protection of argon, weigh 498g (12.76moL) NaNH 2 Add 6kg (12.76moL) 8-hydroxyoctyltriphenylphosphine salt dissolved in 15L organic solvent (3L DMF and 12L hexane) in batches to the reactor, control the temperature not to exceed 15°C, and react for 1h. Take 1.02kg (25.5moL) NaH (content 60%) and continue to add it to the above reaction kettle in batches to disperse it, stir until the system turns dark red, then cool the reaction solution to -15°C, and slowly add 1.1kg (15.26 mol) dilute n-butyraldehyde with 15L organic solvent (3L DMF and 12L hexane), after the dropwise addition is complete, continue to maintain the reaction until the deep red color fades, and the reaction ends. Pour the reaction solution into ice water to inactivate, adjust the pH to neutral with hydrochloric acid, extract with hexane, dry, concentrate the organic phase, and distill the crude product under reduced pressure to obtain the target product 8(Z / E)-dodecen-1-ol 2.176kg, this step y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com