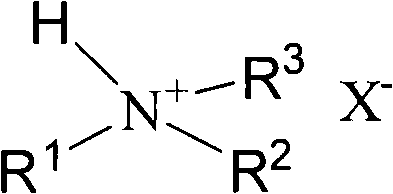
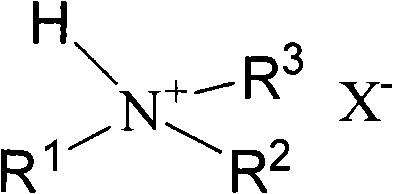
Method for preparing 5-hydroxymethylfurfural
A technology of hydroxymethylfurfural and C2H5, which is applied in the field of preparation of 5-hydroxymethylfurfural, can solve the problems of unfriendly environment, unacceptable cost and low selectivity of the catalytic system, and achieve environmental friendliness, low price and high selectivity. Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Heat 1g of trimethylamine hydrochloride and 0.5g of fructose to 110°C in a preheating dissolving system, and react for 70 minutes (min). After the reaction, the reaction mixture is lowered to room temperature and diluted with water. The method of liquid phase analyzes its productive rate, chromatographic condition: C 18 Column, column temperature 35°C, methanol at volume ratio 1:4 as mobile phase, flow rate 0.6ml / min, UV detector 284nm. Yield 70.5%.
Embodiment 2
[0030] Heat 1g of dimethylamine hydrochloride and 0.6g of fructose to 120°C in a preheating dissolving system, and react for 70 minutes. After the reaction, the reaction mixture is lowered to room temperature and diluted with water. The method determined that the yield was 69.9%.
Embodiment 3
[0032] Heat 1g of diethylamine hydrochloride and 0.55g of fructose in a preheating dissolving system to 120°C and react for 70 minutes. After the reaction, the reaction mixture is lowered to room temperature and diluted with water. The method determined that the yield was 61.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com