Production method of 1,1,1,3,3-pentachlorobutane
A technology of pentachlorobutane and a production method, which is applied in the field of continuous reaction synthesis, can solve the problems of difficult continuous quantitative addition to a reaction system, complicated separation process, and difficult industrialized implementation, etc., and achieves easy implementation, volume reduction, and easy recycling. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
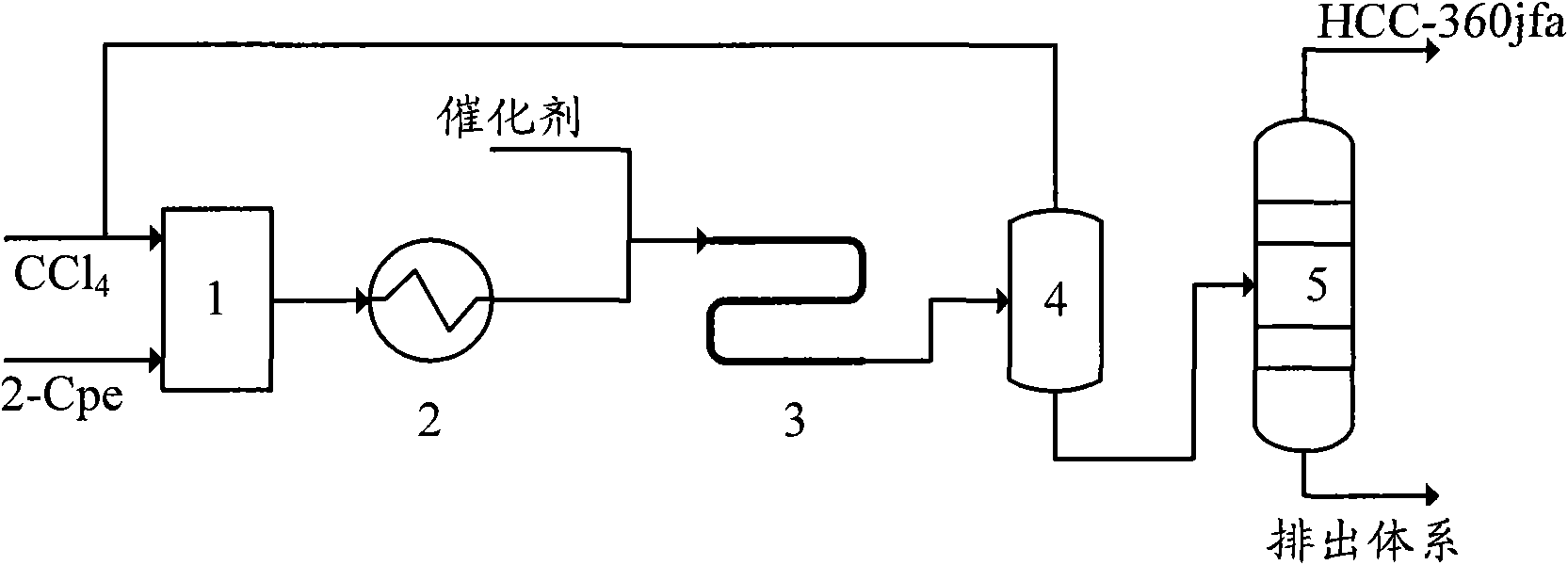
[0037] In this embodiment, the telomerization reactor 4 adopts an externally heated tubular reactor with a pipe diameter of ∮25×2.5 mm and a length of 2 meters. The process flow is as follows:
[0038] A, carbon tetrachloride and 2-chloropropene are mixed in a molar ratio of 10:1, and preheated to 150°C;
[0039] B. Preheated carbon tetrachloride and 2-chloropropene enter the tubular reactor together with the catalyst, and carry out telomerization reaction under the conditions of reaction pressure 1.5MPa, reaction temperature 140°C, and residence time of 5 minutes, wherein the catalyst by FeCl 2 , tributyl phosphate and carbon tetrachloride, FeCl 2 , the molar ratio of tributyl phosphate and carbon tetrachloride is 1:10:20, FeCl 2 The molar ratio to 2-chloropropene is 1:100;
[0040] C, telomerization reaction product removes unreacted carbon tetrachloride through flash evaporation, and unreacted carbon tetrachloride is recycled;
[0041] D. The reaction solution after remov...
Embodiment 2
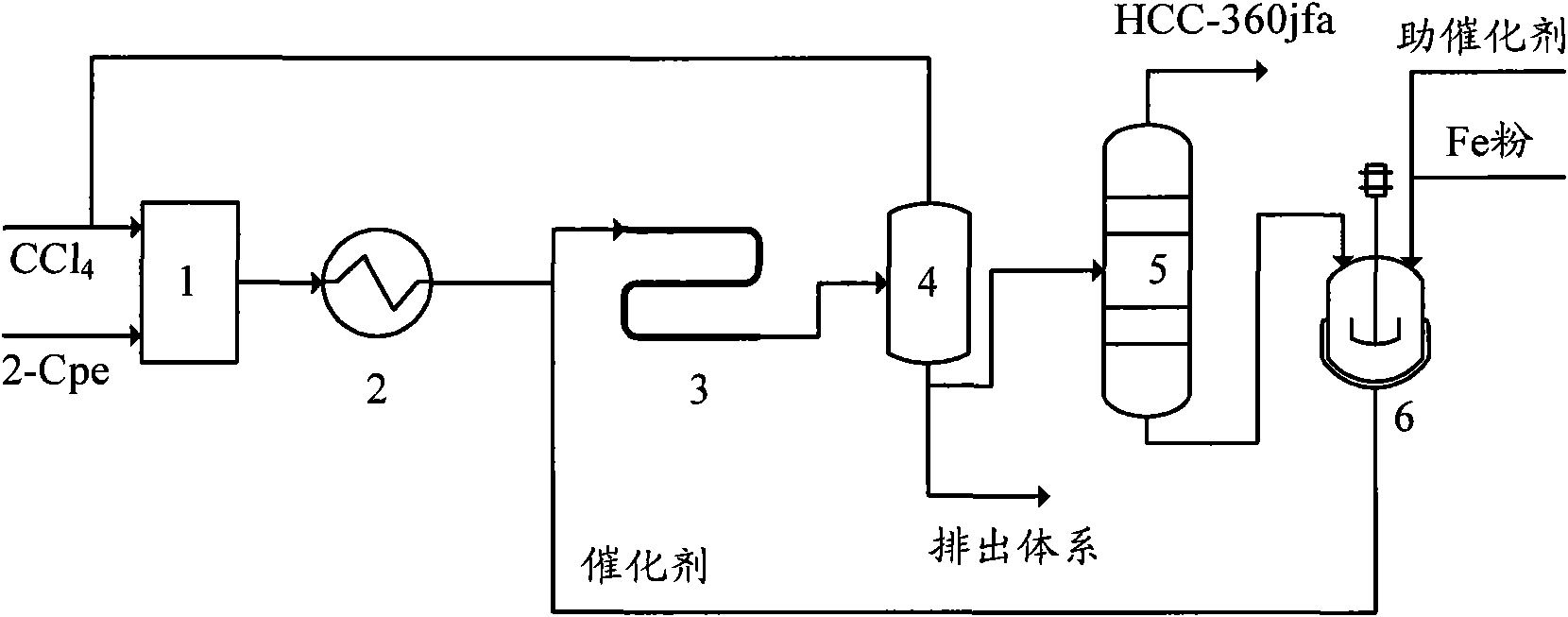
[0044] In this embodiment, the telomerization reactor 3 adopts an adiabatic tubular reactor with a pipe diameter of ∮25×2.5 mm and a length of 2 meters. The process flow is as follows:
[0045] A. Mix carbon tetrachloride and 2-chloropropene in a molar ratio of 1:1, and preheat to 100°C;
[0046] B, preheated carbon tetrachloride and 2-chloropropene enter the tubular reactor together with the catalyst, and carry out the telomerization reaction under the conditions of reaction pressure 0.3MPa, reaction temperature 90°C, and residence time of 60 minutes, wherein The initial catalyst consists of FeCl 2 , triethyl phosphate and carbon tetrachloride, FeCl 2 , the molar ratio of triethyl phosphate and carbon tetrachloride is 1:1:50, and the catalyst is made of FeCl during circulation 2 , triethyl phosphate and telomerization reaction product distillation raffinate; FeCl 2 The molar ratio with 2-chloropropene is 1:20;
[0047] C, telomerization reaction product removes unreacted ...
Embodiment 3
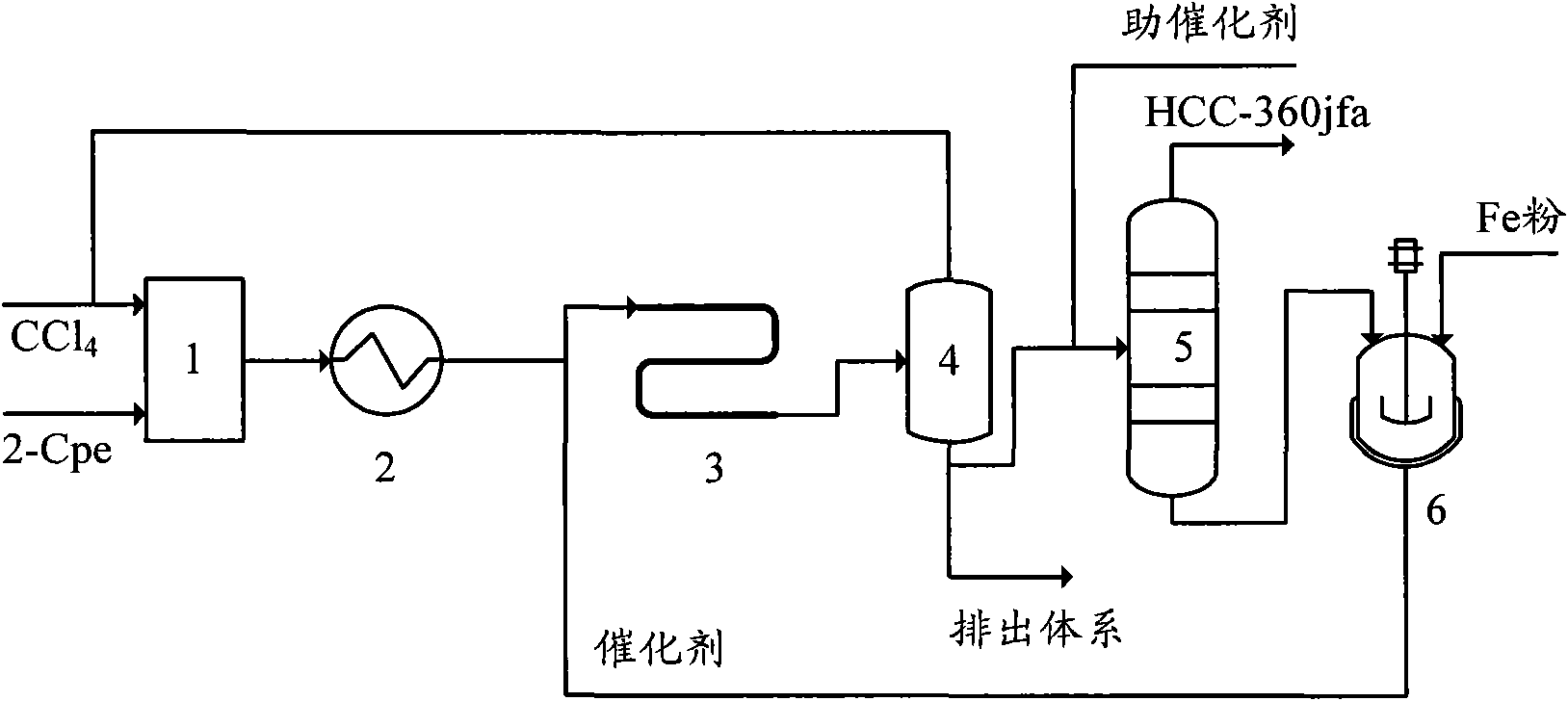
[0053] In this embodiment, the telomerization reactor 4 adopts a Teflon-lined adiabatic tubular reactor with a pipe diameter of ∮38×2.5 mm and a length of 2 meters. The process flow is as follows:
[0054] A, carbon tetrachloride and 2-chloropropene are mixed in a molar ratio of 1.5: 1, and preheated to 130°C;
[0055] B. Preheated carbon tetrachloride and 2-chloropropene enter the tubular reactor together with the catalyst, and carry out telomerization reaction under the conditions of reaction pressure 0.6MPa, reaction temperature 120°C, and residence time of 30 minutes. when the catalyst consists of FeCl 2 , triethyl phosphite and carbon tetrachloride, FeCl 2 , the molar ratio of triethyl phosphate and carbon tetrachloride is 1:3:20, and the catalyst is made of FeCl during circulation 2 , triethyl phosphite and distillation raffinate of telomerization reaction product; FeCl 2 The molar ratio with 2-chloropropene is 1:50;
[0056] C, telomerization reaction product remove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com