Monoclonal antibody against human alpha1 acid glycoprotein and preparation method thereof
A technology of monoclonal antibody and glycosylation, which is applied in the direction of anti-animal/human immunoglobulin, botanical equipment and methods, biochemical equipment and methods, etc. It can solve the problem of poor specificity of antigenic glycoprotein antibodies and complicated experimental operations To achieve the effect of increasing the production of antibodies, enhancing the immune response, and improving the production of antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The gene cloning of embodiment 1 glycoprotein
[0020] The inventors designed PCR primers according to the published gene sequence (SEQ ID NO.1) of human AGP:
[0021] AGP 5' Forward Primer
[0022] 5'-GCCAAGCTTATGGCGCTGTCCTGGGTTCT-3' (SEQ ID ON.2)
[0023] AGP 3' reverse primer
[0024] 5'-GGCGGATCCTAGGATTCCCCCTCCTC-3' (SEQ ID ON.3)
[0025] Using the human liver cDNA library as a template, the full-length AGP gene was amplified by PCR (PCR parameters: 95°C for 5 minutes; 94°C for 30s, 54°C for 30s, 72°C for 1 minute, a total of 40 cycles; 72°C and then extended for 10 minutes). After Hind III and BamH I double digestion, it was connected to the pLNCX2-RFP plasmid (the RFP gene came from the pDsRed2 plasmid, and after the RFP gene was cut out from the plasmid with Hind III and Not I endonucleases, it was connected to the pLNCX2-RFP plasmid after the same double digestion pLNCX2 plasmid), chemically transformed competent cells E.coli DH5α, picked a single clone to e...
Embodiment 2
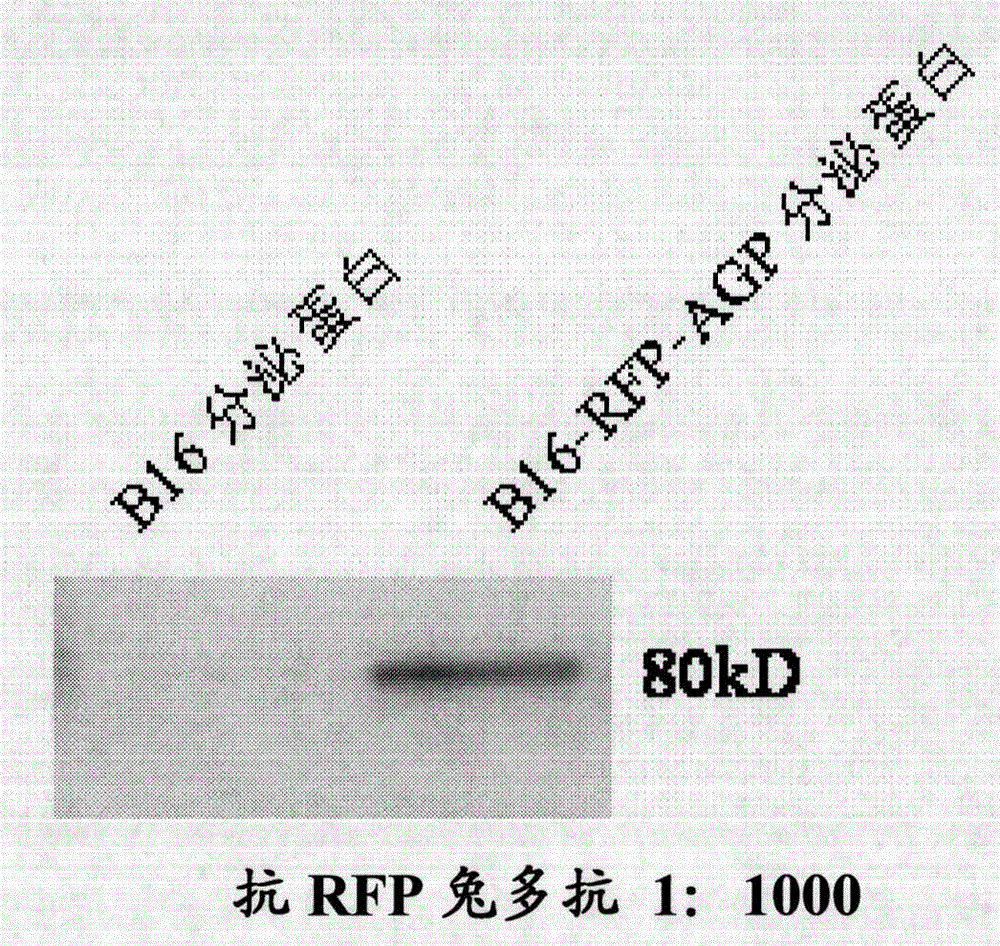
[0026] Example 2 Construction, screening and verification of cell lines stably expressing and secreting exogenous glycoproteins
[0027] Extract the constructed pLNCX2-RFP-AGP plasmid and the plasmid pVSV-G encoding retroviral envelope protein from E.coli DH5α, according to the amount of DNA and transfection reagent Lipofectamine2000 (11668-019, Invitrogen) is 1:5 The ratio of the two plasmids was co-transfected. With the help of chloroquine, the two plasmids were transformed into retroviral packaging cells GP2-293. After 8 hours, the medium was changed to eliminate the toxic effect of chloroquine on the cells. 48 hours after the withdrawal of chloroquine, the culture supernatant of GP2-293 was collected, and the retroviral particles containing foreign genes were released in the culture medium at this time. The precipitated retroviral particles were enriched by prolonged high-speed centrifugation (4°C, 50,000g, 90 minutes). After renatured, these viruses were added to the c...
Embodiment 3A
[0028] The preparation of embodiment 3AGP recombinant antigen
[0029] Design PCR primers according to the published gene sequence of human AGP:
[0030] AGP 5' Forward Primer
[0031] 5'-GCCGGATCCATGGCGCTGTCCTGGGTTCT-3' (SEQ ID ON.4)
[0032] AGP 3' reverse primer
[0033] 5'-GGCAAGCTTCTAGGATTCCCCCTCCTC-3' (SEQ ID ON.5)
[0034] Using the human liver cDNA library as a template, the full-length AGP gene was amplified by PCR, and then double-digested with BamH I and Hind III after recovery from the gel. After recovery, it was ligated with the same double-digested pET28a vector, and chemically transformed into competent cells E.coli DH5α, positive clones were selected after double enzyme digestion verification and plasmid sequencing. The plasmids of the selected positive clones were chemically transformed into competent cells BL21(DE3). Inoculate the BL21 strain that can express AGP, and when the OD value of the bacterial solution reaches about 0.6, add 1 mmol / L IPTG for in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com