Organosilane coating compositions and use thereof
A coating composition and organosilane technology, applied in coatings, metal material coating processes, anti-corrosion coatings, etc., can solve problems such as high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0224] Example 1 ——Synthesis of Disubstituted Tetrazine
[0225] 1.1 Synthesis of 3,6-bis(3,5-dimethylpyrazol-1-yl)-1,2,4,5-tetrazine (dmptz)
[0226] The synthesis was carried out using a published three-step procedure (Codburn et al., 1991) according to the following scheme:
[0227]
[0228] 1.1.1 Synthesis of triaminoguanidine monohydrochloride
[0229] Hydrazine monohydrate was slowly added to a solution of guanidine hydrochloride (19.1 g, 0.020 mol) in 1,4-dioxane (100 ml) under stirring. The resulting mixture was heated to reflux for 2h. The resulting solution was then cooled to room temperature and the product was collected by Buchner filtration, washed with 1,4-dioxane, and dried in air. The product formed is triaminoguanidine.
[0230] 1.1.2 Synthesis of 3,6-bis(3,5-dimethylpyrazol-1-yl)-1,2-dihydro-1,2,4,5-tetrazine
[0231] To an aqueous solution (150 ml) of triaminoguanidine monohydrochloride (21.09 g, 0.15 mol) cooled in an ice bath was slowly added...
Embodiment 2
[0267] Example 2 - Formulation of sol-gels containing ligands as chelating agents
[0268] In this example, methacrylic acid (MAAH) was used as the chelating agent.
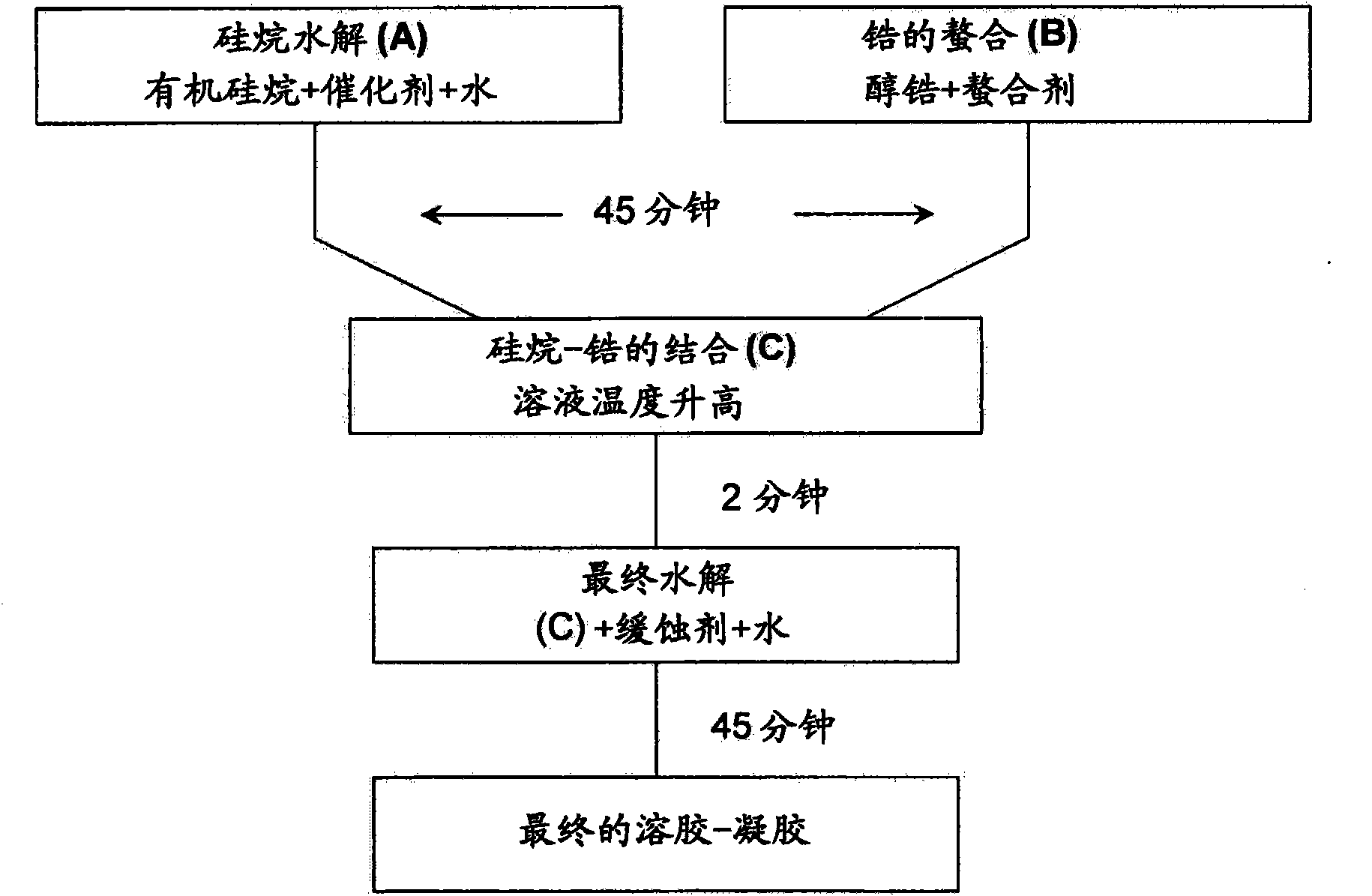
[0269] see figure 2 The flow chart:
[0270] Hydrolysis of organosilanes (A)
[0271] By using 0.01M HNO 3 Hydrolysis of organosilanes was carried out by hydrolyzing MAPTMS in aqueous solution at a volume ratio of 1:0.75 below which precipitation of zirconium species occurred during the second hydrolysis. Due to the immiscibility of MAPTMS and water, the hydrolysis proceeds in a heterogeneous manner. After stirring for 20 minutes, enough methanol had been produced to make everything present in the solution miscible.
[0272] Zirconium Chelation (B)
[0273]In order to control the hydrolytic condensation reaction, strong complexing ligands are usually used for silicate-free metal alkoxide precursors (Livage and Sanchez, 1992). Among these strong complexing ligands, MAAH can be covalently bonded to th...
Embodiment 3
[0279] Example 3 - Preparation of sol-gel containing the corrosion inhibitor as a chelating agent
[0280] In this example, DPTZ was used as the chelating agent.
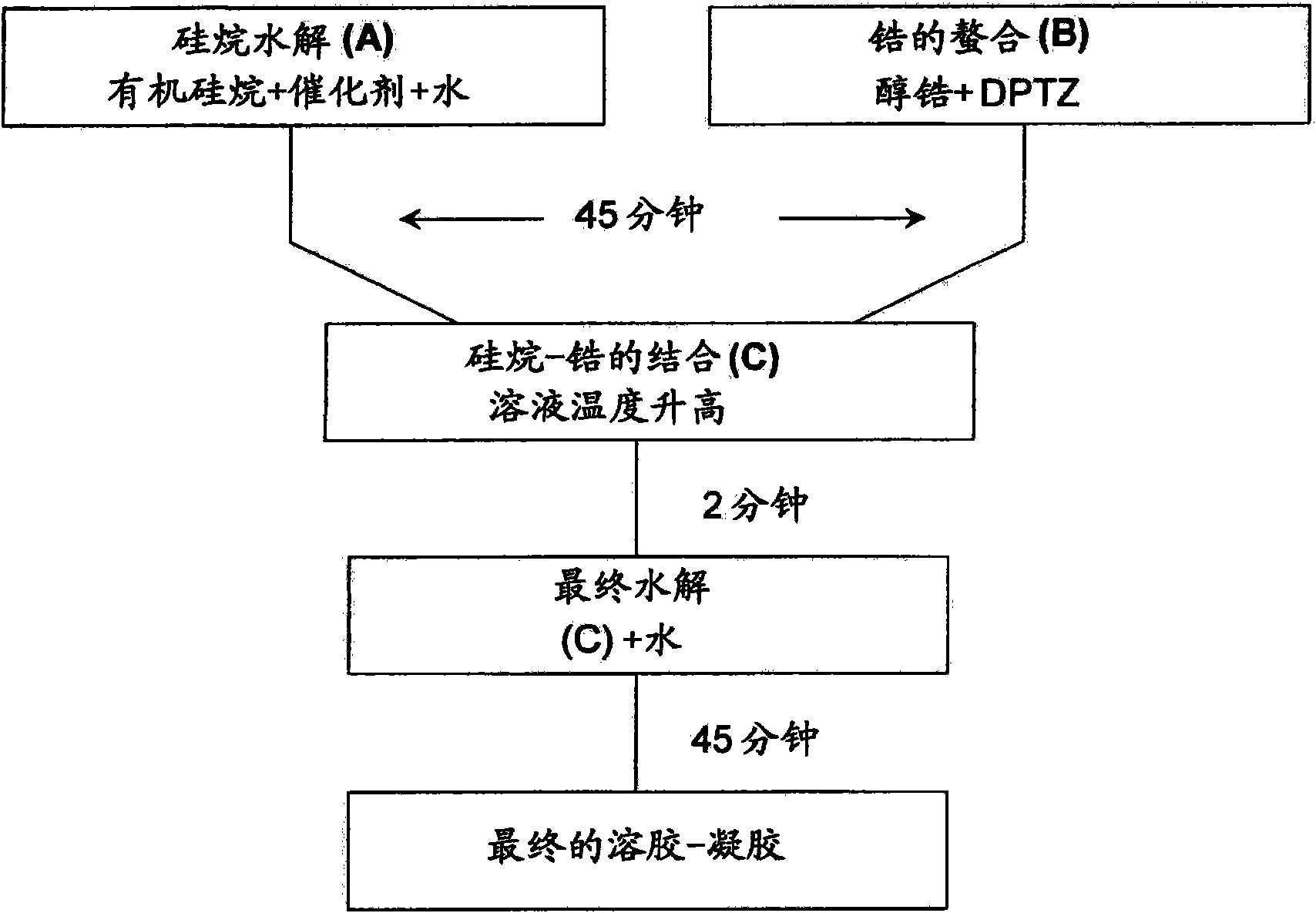
[0281] see image 3 :
[0282] Organosilane hydrolysis (A)
[0283] With 0.01M HNO 3 The aqueous solution hydrolyzed MAPTMS at a volume ratio of 1:0.75 to carry out the hydrolysis of organosilanes. Due to the immiscibility of MAPTMS and water, the hydrolysis proceeds in a heterogeneous manner. After stirring for 20 minutes, enough methanol had been produced to make everything present in the solution miscible.
[0284] Zirconium Chelation (B)
[0285] to Zr(OPr) at a molar ratio of 1:1 4 DPTZ (dissolved in EtOH) was added dropwise.
[0286] Combination of organosilane and zirconium (C)
[0287] After about 45 minutes (using the concentrations above), the partially hydrolyzed MAPTMS was slowly added to the zirconate complex. This mixture was characterized by an increase in temperature, demonstrating ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com