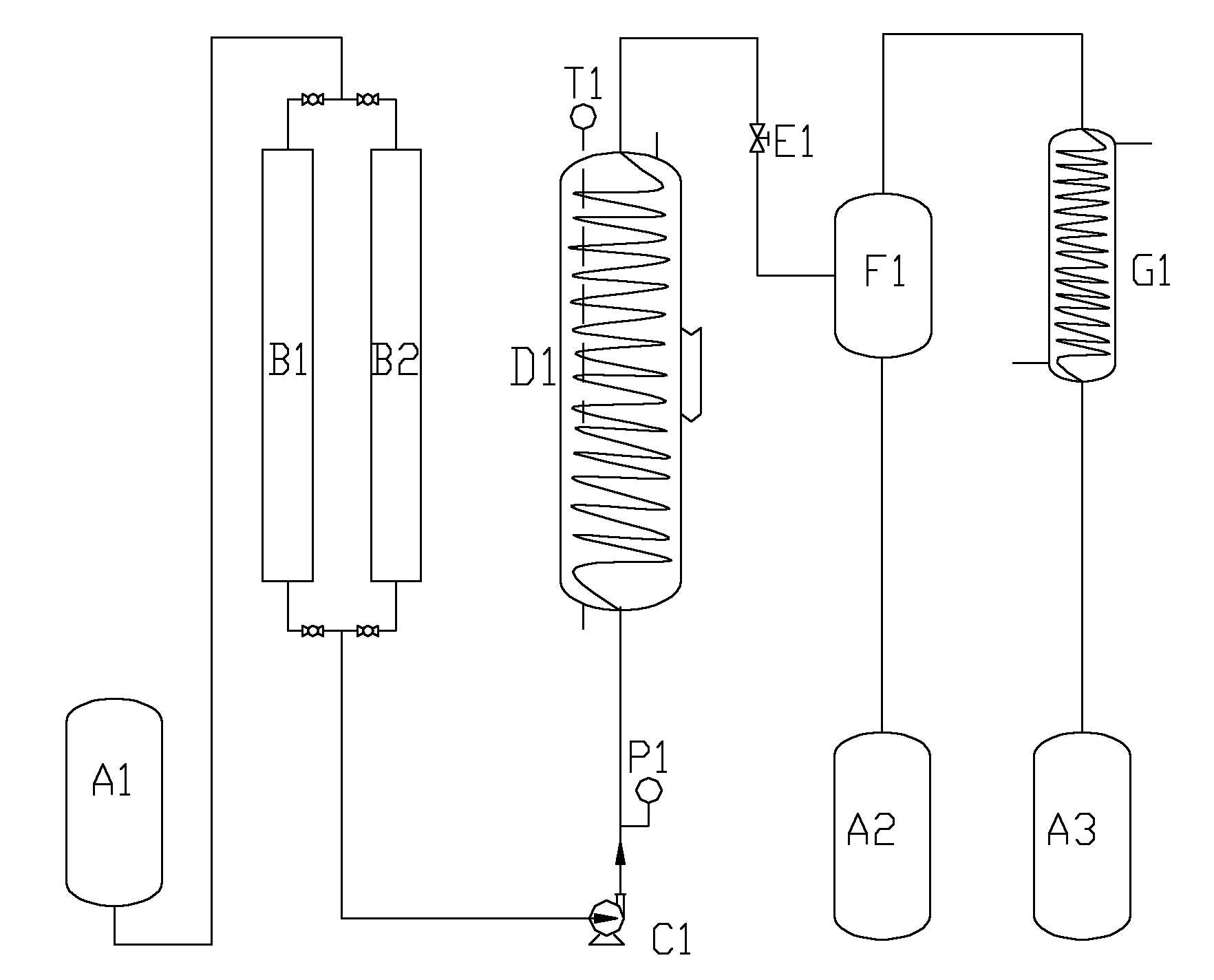
Method for supercritically synthesizing leaf alcohol
A supercritical, leaf alcohol technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of hydroxyl compounds, etc., can solve the problems of high corrosion of equipment, harsh reaction conditions, poor atom economy, etc., and achieve good heat transfer performance. , stable operation, low pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10
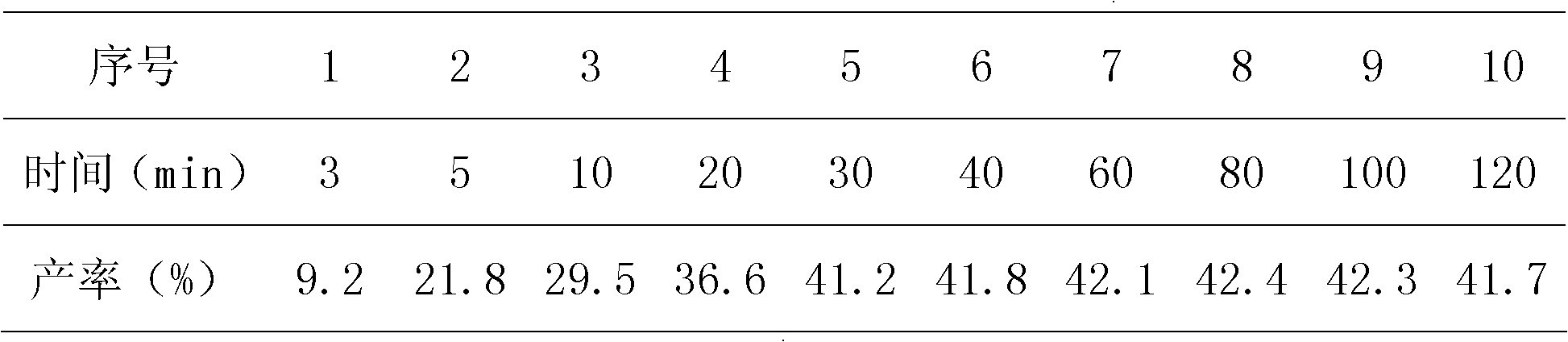
[0018] In the supercritical, continuous reaction of n-pentene / formaldehyde in methanol solution under catalyst-free conditions, the yields of different residence times and 3-hexen-1-alcohol relative to formaldehyde are shown in Table 1.
[0019] Table 1 residence time and the relation of the productive rate of 3-hexen-1-alcohol relative formaldehyde
[0020]
[0021] Reaction conditions: n-pentene: formaldehyde = 6 (mol); temperature, 280°C; pressure, 20MPa.
example 11-20
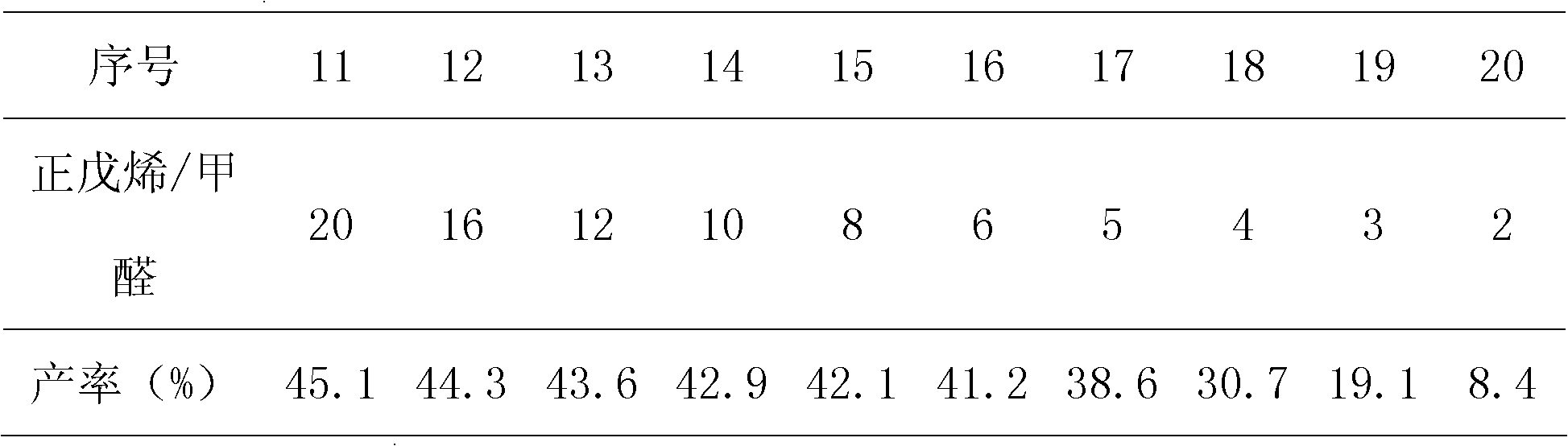
[0023] See Table 2 for the relation between the mol ratio of raw materials and the productive rate of 3-hexen-1-alcohol relative to formaldehyde.
[0024] The relation of the mol ratio of table 2 raw material and the productive rate of 3-hexen-1-alcohol relative formaldehyde
[0025]
[0026] Reaction conditions: temperature, 280°C; pressure, 20MPa; residence time, 30min.
Embodiment 21-30
[0028] Reaction temperature and 3-hexen-1-ol relative formaldehyde productive rate are shown in Table 3.
[0029] The relation of table 3 reaction temperature and the productive rate of 3-hexen-1-alcohol relative formaldehyde
[0030]
[0031] Reaction conditions: n-pentene: formaldehyde = 6 (mol); pressure, 20MPa, residence time, 30min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com