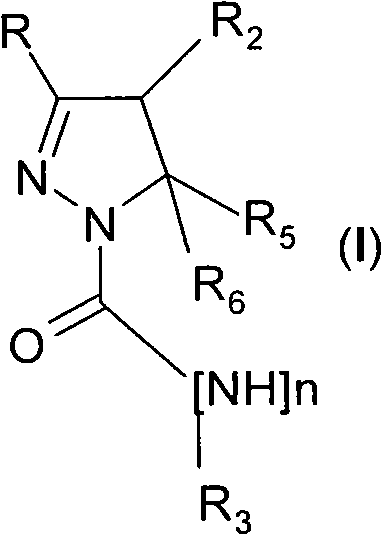
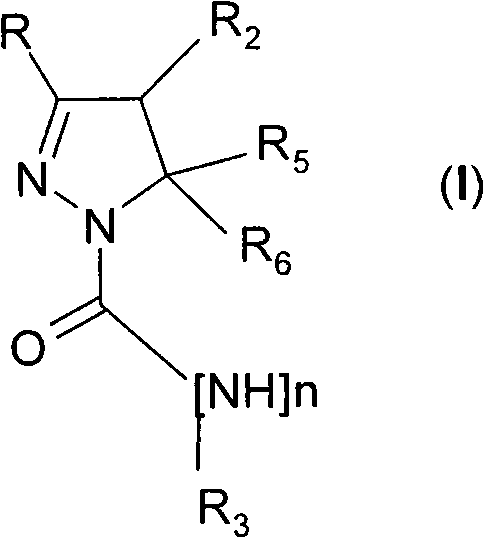
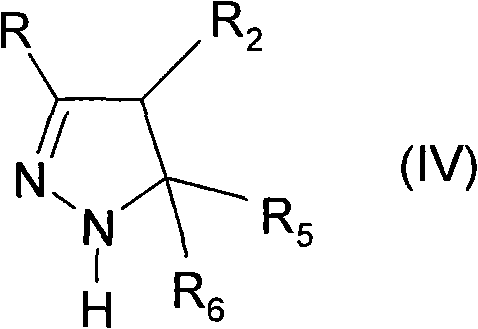
4,5-dihydro-(1h)-pyrazole derivatives as cannabinoid CB1 receptor modulators
A technology of derivatives and compounds, applied in the fields of medicine and organic chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Example 1: Materials and methods
[0117] On Varian 300MHz instrument, Varian UN400 instrument (400MHz), use DMSO-d 6 or CDCl 3 As a solvent, using tetramethylsilane as an internal standard, record 1 H NMR spectrum. Using CDCl on a Varian UN400 instrument 3 As a solvent, record 13 C NMR spectrum. Chemical shifts are given in ppm (delta scale) downfield from tetramethylsilane. Coupling constants (J) are expressed in Hz. Flash chromatography was performed using silica gel 60 (0.040-0.063 mm, Merck). Column chromatography was performed using silica gel 60 (0.063-0.200 mm, Merck). Using Supelco equipment, VersaFLASH TM Column, VersaPak TM Silica cartridges, Büchi UV Monitor C-630, Büchi Pump Module C-605, Büchi Fraction Collector C-660 and Büchi Pump Manipulator C-615 were used for Sepacore chromatographic separation. Melting points were recorded on a Büchi B-545 melting point apparatus or determined by DSC method. The optical rotation ([α] D ). Optical rotati...
Embodiment 2
[0118] Example 2: General aspects of synthesis
[0119] Pyrazoline derivatives can be prepared by published methods (Barluenga, 1999 (and references cited therein); Wang, 2003). In Scheme 1 the synthesis of compounds of general formula (I) is outlined. Ketone derivatives of general formula (II) can be prepared by various methods known to those skilled in the art. An example is the Weinreb amide RC(=O)N(OCH 3 )CH 3 application, which can be used with the Grignard reagent R 2 CH 2 MgCl or R 2 CH 2 MgBr reacts, or RMgBr or RMgCl with the general formula R 2 CH 2 C(=O)N(OCH 3 )CH 3 The Weinreb amide reaction. Alternatively, Grignard reagent R 2 CH 2 MgCl or R 2 CH 2 MgBr can be reacted with HCN followed by acidic hydrolysis, for example by using hydrochloric acid. where R and R 2 The ketone derivative of the general formula (II) having the above-mentioned meaning can be in the presence of a base, such as sodium methylate, with the general formula R 5 R 6 The hal...
Embodiment 3
[0134] Example 3: Synthesis and Spectral Data of Intermediates
[0135] Intermediate II-1
[0136]
[0137] Part A: To a magnetically stirred solution of valeric acid (10.9ml, 0.1mol) in dichloromethane (200ml) was added sequentially: N-methyl-N-methoxy-amine.hydrochloride (10.14g, 0.104mol), N-methylmorpholine (22.9ml, 0.208mol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide.HCl (EDCl) (19.92 grams, 0.104mol ) and HOBt (14.04 g, 0.104 mol) and the resulting mixture was reacted at room temperature for 20 hours. Water, citric acid aqueous solution (24 grams of citric acid in 250ml H 2 solution in O) and 5% NaHCO 3 The obtained suspension was washed with aqueous solution. in Na 2 SO 4 The organic layer was dried on top, filtered and concentrated to afford methoxy-methyl-valeric acid (12.31 g, 85% yield) as a colorless oil. 1 H-NMR (400MHz, CDCl 3 )δ0.93(t, J=7, 3H), 1.31-1.43(m, 2H), 1.57-1.66(m, 2H), 2.42(br t, J=7, 2H), 3.18(s, 3H) , 3.69(s, 3H).
[0138] Part B: ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com