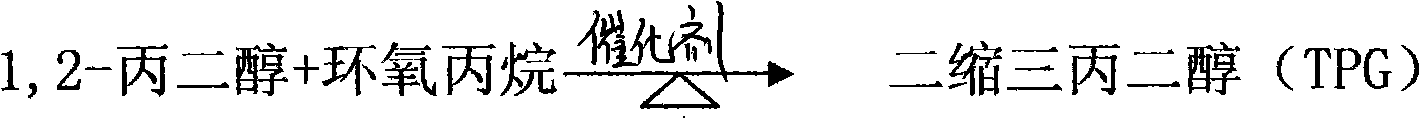Method for synthesizing tripropylene glycol
A technology for the synthesis of tripropylene glycol and its synthesis method, which is applied in the synthesis of tripropylene glycol and the synthesis field of tripropylene glycol, and can solve the problems of inability to use high-grade light-colored paint and ink, low monomer ester content, and low product yield, etc. problems, to achieve the effect of shortened reaction time, less impurities and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 1,2 propylene glycol 500g, monohydrate barium hydroxide 4.0g in the reaction kettle, replace N 2 After three times, the temperature was raised to 85°C for dehydration for 1 hour, and then the temperature was raised to 85°C to add 573 g of propylene oxide. After the reaction was completed for 2 hours, the temperature was lowered for degassing. Add 2.1 g of sulfuric acid for neutralization, filter and discharge to obtain tripropylene glycol. The reaction pressure is -0.05Mpa. Gas chromatographic analysis: the content of tripropylene glycol is 48.5%, and pentapropylene glycol cannot be detected. Visually inspect the sample for color No. 5 (Hazen units).
Embodiment 2
[0019] Add 1,2 propylene glycol 500g, octahydrate barium hydroxide 12.0g in the reaction kettle, replace N 2 After three times, the temperature was raised to 85°C for dehydration for 1 hour, and then the temperature was raised to 100°C to continue adding 690 g of propylene oxide. The reaction temperature was 100-105°C. After the reaction was completed for 2 hours, the temperature was lowered for degassing. Add 3.8 g of sulfuric acid for neutralization, filter and discharge to obtain tripropylene glycol. The reaction pressure is 0.45Mpa. Gas chromatographic analysis: the content of tripropylene glycol is 58.6%, and the content of pentapropylene glycol is 0.35%. Visually inspect the sample for color No. 5 (Hazen units).
Embodiment 3
[0021] Add 1,2 propylene glycol 500g, monohydrate barium hydroxide 10.0g in the reaction kettle, replace N 2 After three times, the temperature was raised to 85°C for dehydration for 1 hour, and then the temperature was raised to 120°C to continue adding 800 g of propylene oxide. The reaction temperature was 120-125°C. After the reaction was completed for 2 hours, the temperature was lowered for degassing. Add 5.28 g of sulfuric acid for neutralization, filter and discharge to obtain tripropylene glycol. The reaction pressure is 0.1Mpa. Gas chromatography analysis: the content of tripropylene glycol is 64.5%, and the content of pentapropylene glycol is 1.0%. Visually inspect the sample for color No. 10 (Hazen units).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com