Veterinary nanometer suspension, preparation method thereof and application thereof
A nanosuspension and veterinary drug technology, applied in the directions of pharmaceutical formulations, antibacterial drugs, drug combinations, etc., can solve the problems of lack of formula, preparation method and application of veterinary drug nanosuspension, and improve the bioavailability and treatment of veterinary drugs. effect, reducing dosage, reducing the effect of organic solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
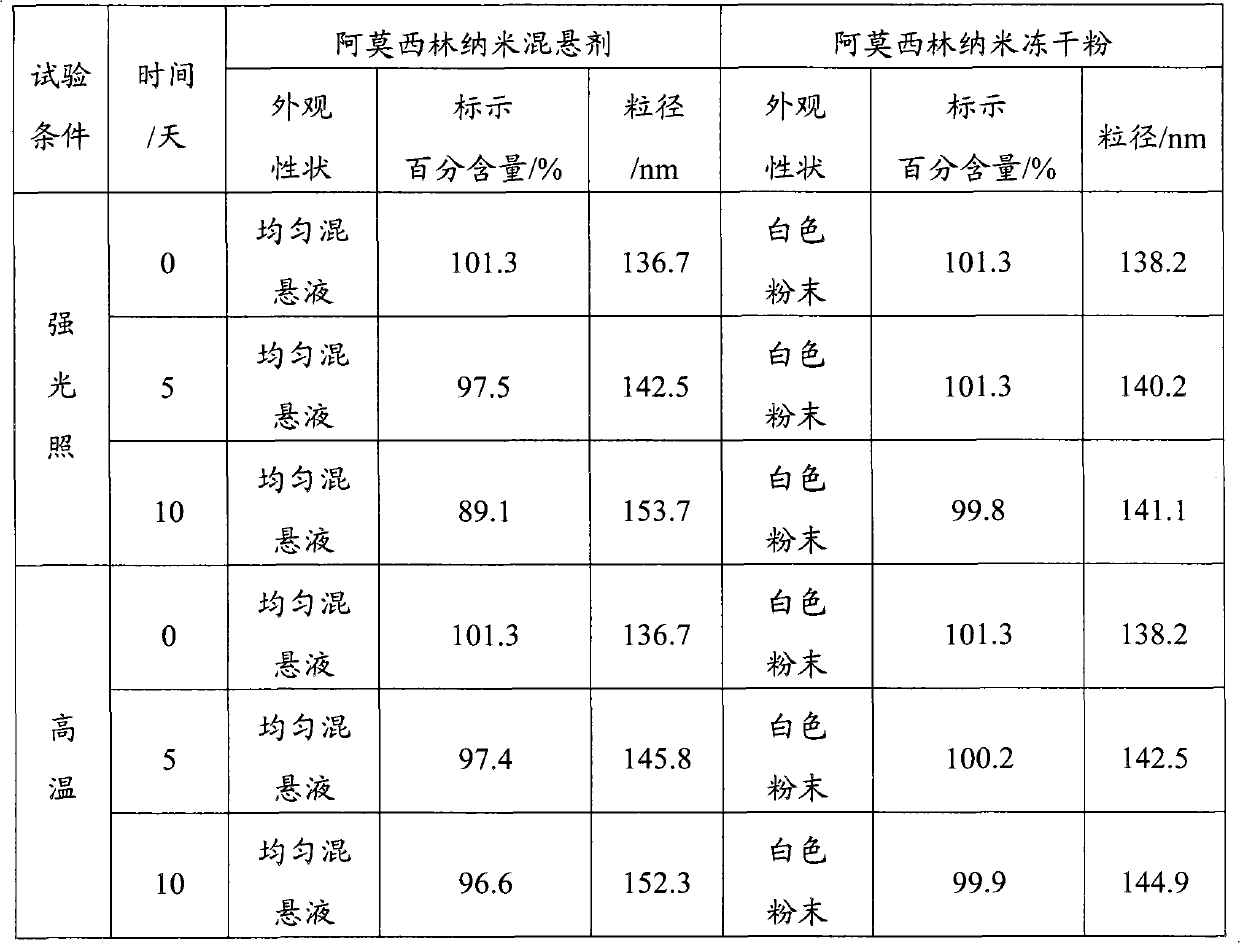
[0034] The preparation method of the nanosuspension of the present invention uses a high-pressure homogenizer to repeat homogenization and microporous filtration, and the particle diameter obtained is less, and the particle diameter is less than 220nm, and the particle diameter range difference (the maximum particle diameter of a medicine and The minimum particle size difference) is 45-85 nm, while the ordinary suspension not treated by the method of the present invention has a larger particle size, generally 0-50 μm, and its particle size range is as high as about tens of microns. Therefore, the nanosuspension obtained by the method of the present invention has a small particle size, which is convenient for absorption by animal bodies.
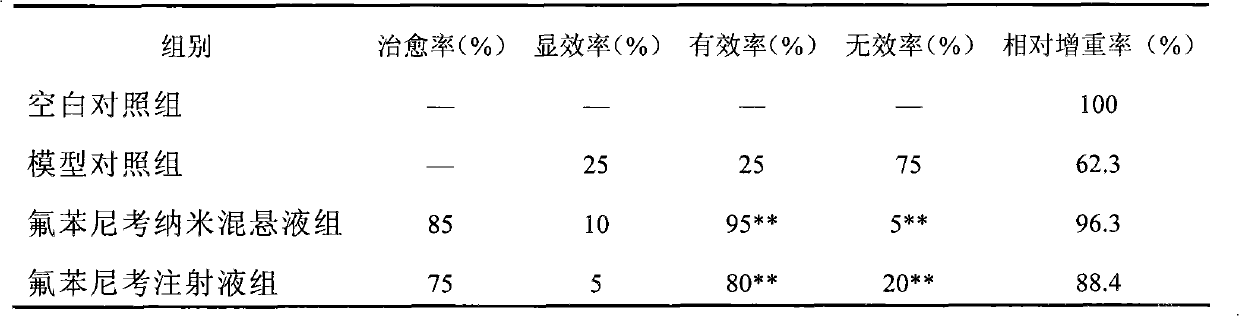
[0035]Example 1 of the present invention was carried out by measuring the particle size, stability, irritation and clinical curative effect of the florfenicol nanosuspension prepared by the present invention. As a result, the nano-suspension ...
Embodiment 1
[0040] Preparation and properties of embodiment 1 Florfenicol injection nanosuspension
[0041] 1. Preparation of Florfenicol Injectable Nanosuspension
[0042] Measure the reagents and drugs according to the following proportions:
[0043] Florfenicol 30% W / V
[0044] Povidone 20% W / V
[0045] Propylene Glycol 10%W / V
[0046] Phospholipids 5%W / V
[0047] Sodium sulfite 0.2%W / V
[0048] Water for injection to 100% volume.
[0049] The specific process of preparation is:
[0050] 1) Take 300ml of water for injection, add 100g of propylene glycol, mix well, add 300g of florfenicol, stir well to obtain a mixture;
[0051] 2) Take 200ml of water for injection, add 2g of sodium sulfite, 200g of povidone, and 50g of phospholipid, and stir to obtain a mixture;
[0052] 3) Add the mixture obtained in step 2) to the mixture obtained in step 1), mix well, add water for injection to 1000ml, mix well, then repeat the homogenization through a high-pressure homogenizer for 2-3 times...
Embodiment 2
[0097] Example 2 Amoxicillin injection nanosuspension and freeze-dried powder
[0098] 1. Preparation of Amoxicillin Nanosuspension
[0099] Amoxicillin 15% W / V
[0100] Povidone 15% W / V
[0101] Propylene Glycol 8%W / V
[0102] Phospholipids 3%W / V
[0103] Sodium Thiosulfate 0.1%W / V
[0104] Water for injection to 100% volume.
[0105] The preparation method refers to Example 1 to obtain the amoxicillin nanosuspension.
[0106] 2. Preparation of Amoxicillin Nano-lyophilized Powder
[0107] Take 500ml of amoxicillin nano-suspension, add 2.5g of aspartic acid, and freeze-dry to remove the organic solvent to obtain a sterile freeze-dried solid powder of amoxicillin nanoparticles.
[0108] Amoxicillin nano-suspension and lyophilized powder were measured by laser particle size analyzer, the average particle diameters were 136.7nm and 138.2nm respectively, the particle diameter ranges were 108-153nm, 113-158nm respectively, and the particle diameter range difference was 45nm ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com