Nano-gold catalyst supported on combined metal oxide, preparation method and application thereof
A composite metal and catalyst technology, which is applied in the direction of metal/metal oxide/metal hydroxide catalyst, hydroxyl compound preparation, organic compound preparation, etc. It can solve the problems of difficult control of the preparation process, poor catalytic performance, and large particle size of gold particles. etc. to achieve the effects of controllable hydrogenation selectivity, mild reaction conditions and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
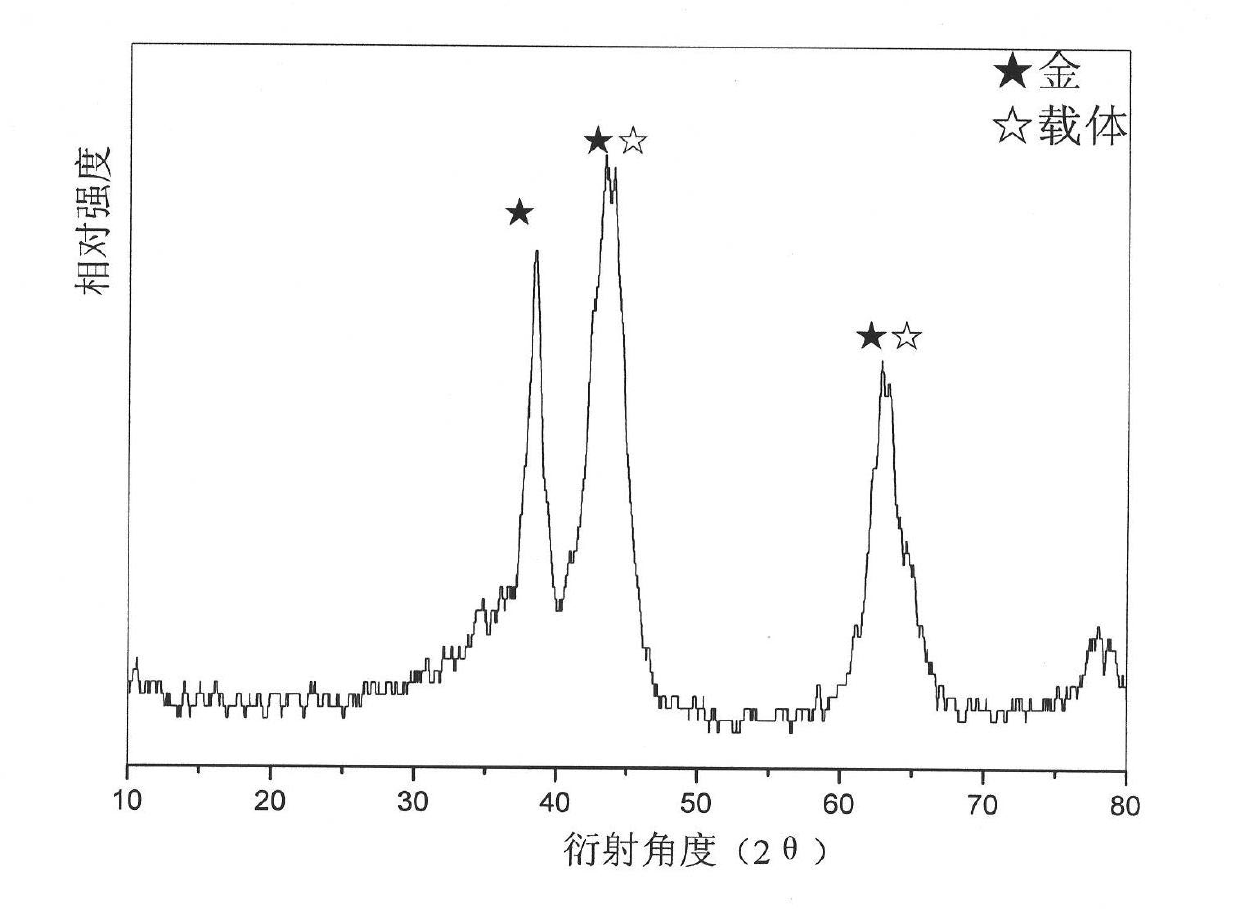
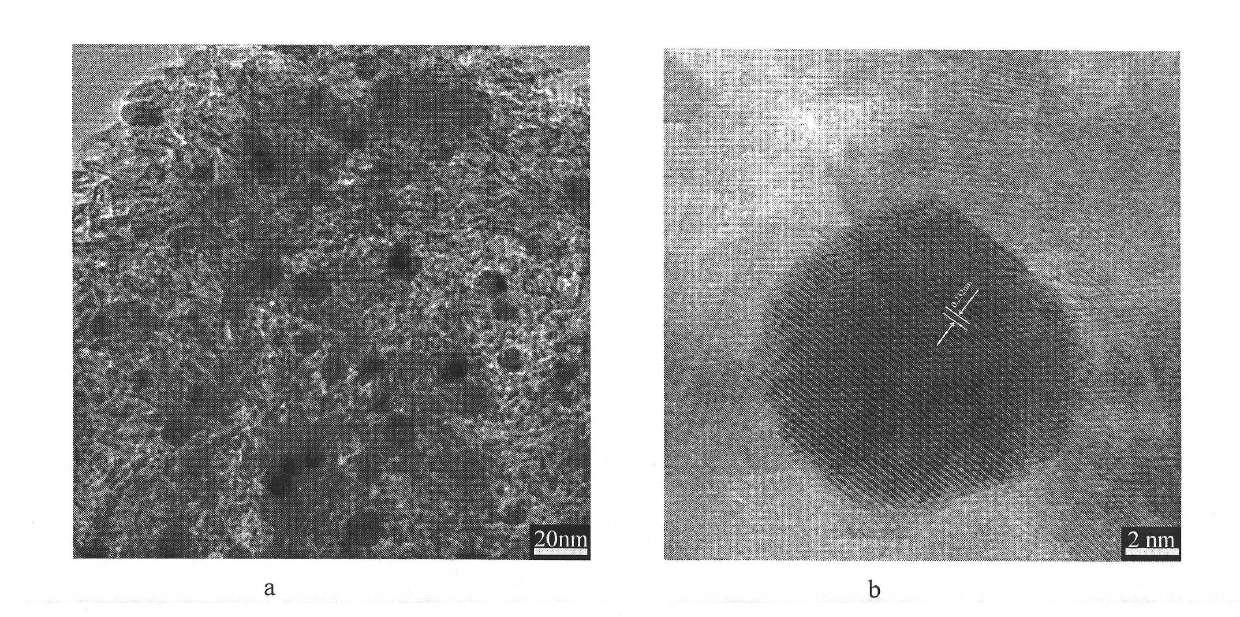
Image
Examples
Embodiment 1
[0021] Weigh 1.5382gMg(NO 3 ) 2 ·6H 2 O and 2.2510gAl(NO 3 ) 3 9H 2 O was dissolved in 30ml deionized water to prepare a mixed salt solution, in which Al 3+ The molar concentration is 0.2mol / L, Mg 2+ The molar concentration of Al 3+ 1 times the molar concentration; with 0.768gNaOH and 1.2718gNa 2 CO 3 Prepare mixed alkali solution 30ml, wherein NaOH molar concentration is (Mg 2+ +Al 3+ ) concentration of 1.6 times, Na 2 CO 3 Concentration is Al 3+ 2 times the concentration.
[0022] Pour the prepared mixed salt solution and mixed alkali solution into the fully back-mixed liquid film reactor at room temperature. Under the condition that the rotating speed of the liquid film reactor is 4200r / min and the gap size between the stator and the rotor is 16 μm, the violent After rotating and stirring for 3 min, the obtained suspension was centrifuged and washed to pH=7 to obtain a precipitate.
[0023]Weigh 0.5g of sucrose and dissolve it in 100ml of deionized water, add...
Embodiment 2
[0027] Weigh 2.3077gMg(NO 3 ) 2 ·6H 2 O and 1.1254gAl(NO 3 ) 3 9H 2 O was dissolved in 30ml deionized water to prepare a mixed salt solution, in which Al 3+ The molar concentration is 0.1mol / L, Mg 2+ The molar concentration of Al 3+ 3 times the molar concentration; weigh 0.768gNaOH and 0.636gNa 2 CO 3 Prepare mixed alkali solution 30ml, wherein NaOH molar concentration is (Mg 2+ +Al 3+ ) concentration of 1.6 times, Na 2 CO 3 Concentration is Al 3+ 2 times the concentration, the prepared mixed salt solution and mixed alkali solution are poured into the full return mixed liquid film reactor at room temperature, the rotating speed of the liquid film reactor is 4200r / min, and the gap size between the stator and the rotor is Under the condition of 16 μm, vigorously rotate and stir for 3 minutes, and centrifuge and wash the obtained suspension to pH=7 to obtain a precipitate. Weigh 0.5g of sucrose and dissolve it in 100ml of deionized water, add the obtained layered do...
Embodiment 3
[0031] Weigh 2.3077gMg(NO 3 ) 2 ·6H 2 O and 1.1254gAl(NO 3 ) 3 9H 2 O was dissolved in 30ml deionized water to prepare a mixed salt solution, in which Al 3+ The molar concentration is 0.1mol / L, Mg 2+ The molar concentration of Al 3+ 3 times the molar concentration; weigh 0.768gNaOH and 0.636gNa 2 CO 3 Prepare mixed alkali solution 30ml, wherein NaOH molar concentration is (Mg 2+ +Al 3+ ) concentration of 1.6 times, Na 2 CO 3 Concentration is Al 3+ 2 times the concentration, the prepared mixed salt solution and mixed alkali solution are poured into the full return mixed liquid film reactor at room temperature, the rotating speed of the liquid film reactor is 4200r / min, and the gap size between the stator and the rotor is Under the condition of 16 μm, vigorously rotate and stir for 3 minutes, and centrifuge and wash the obtained suspension to pH=7 to obtain a precipitate. Weigh 1g of sucrose and dissolve it in 100ml of deionized water, add the obtained layered doub...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
| The average diameter | aaaaa | aaaaa |
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com