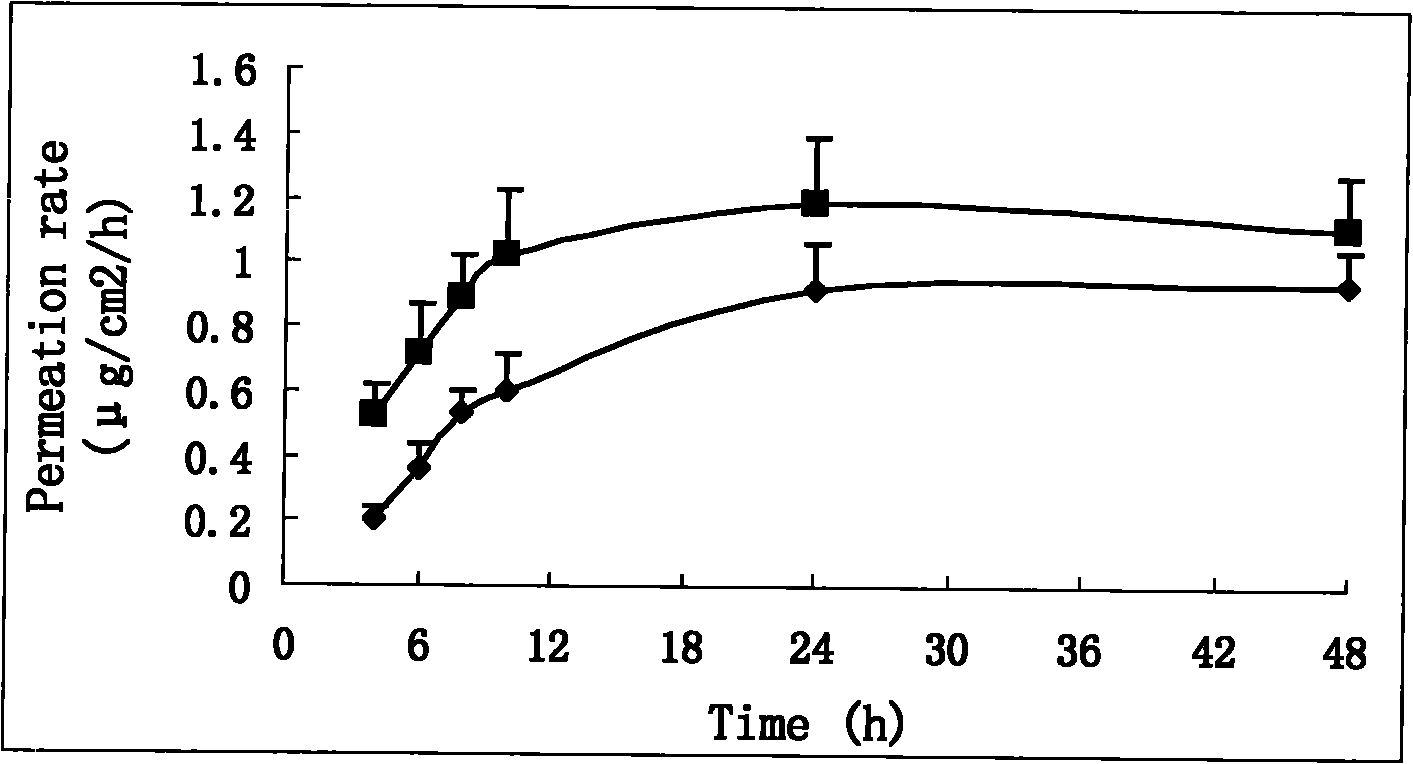
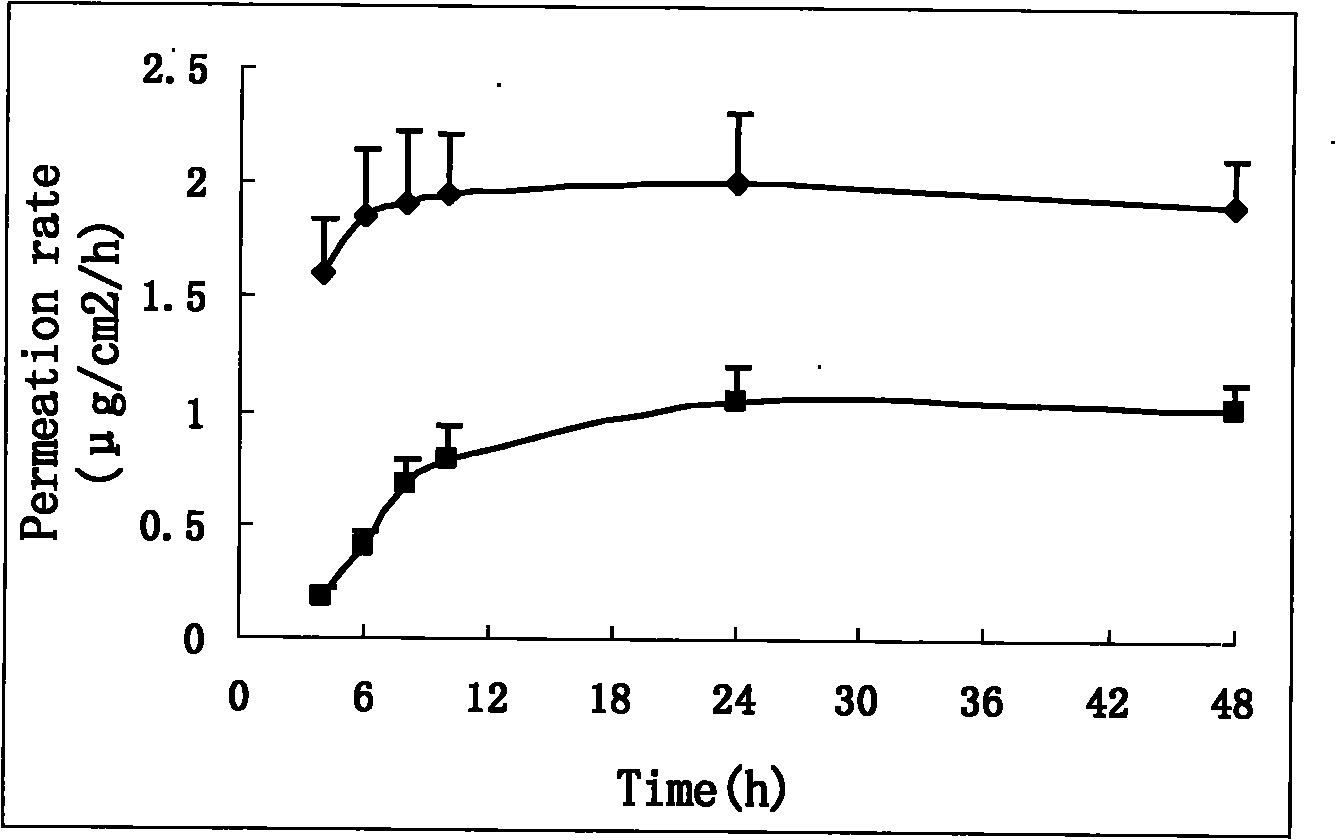
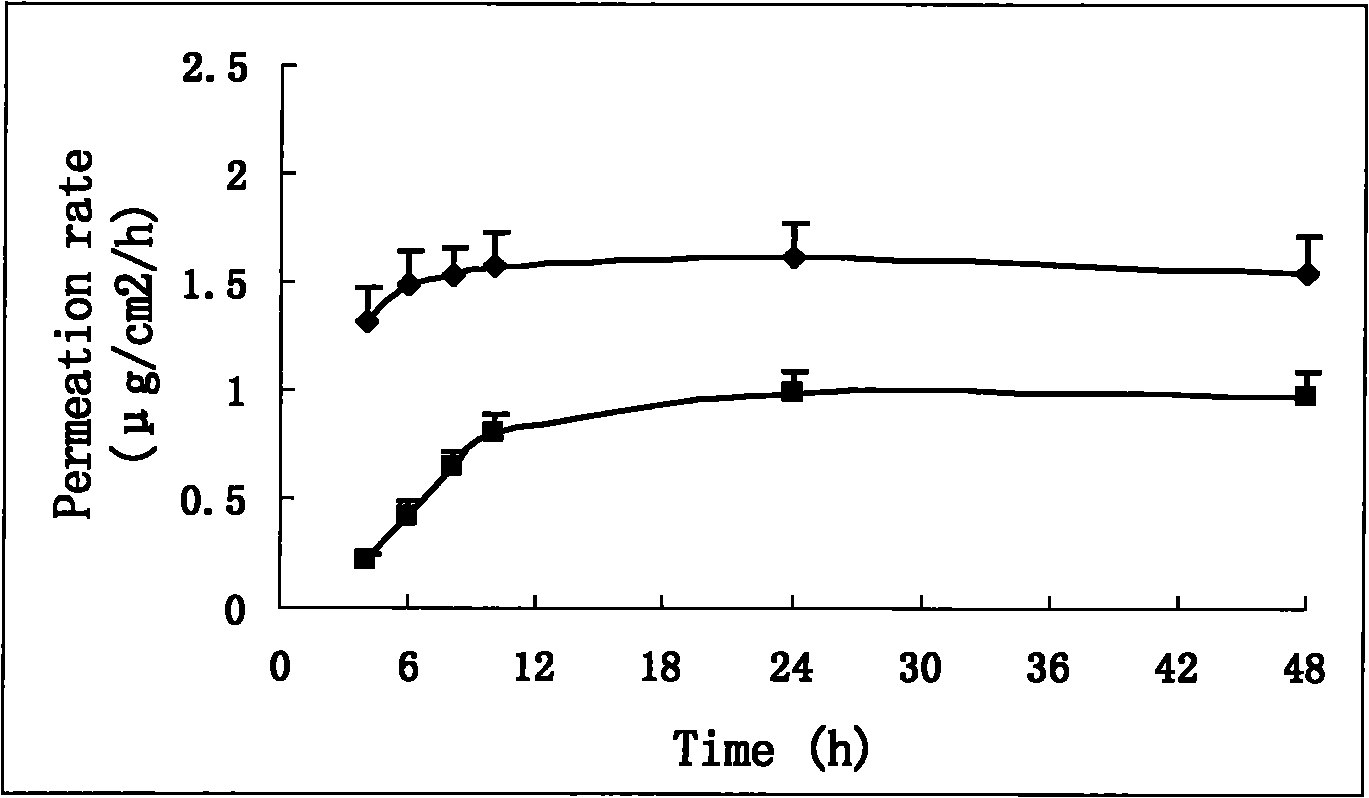
Double-controlled release, high-dosage and low-irritation capsaicin compound transdermal patch
A transdermal patch and high-dose technology, which is applied in the field of medicine, can solve the problems of short tolerance time and poor compliance of high-dose capsaicin, and achieve the effects of promoting drug efficacy, reducing irritation, and improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Prescription (43cm 2 Feeding)
[0041] upper layer:
[0042] Menthol 0.10g
[0043] borneol 0.10g
[0044] Oleic acid (OA) 0.08g
[0045] 1,2-propanediol (PG) 0.20g
[0046] Dibutylphenol 0.01g
[0047] Polyacrylate (87-2852, American Chemical Starch Company) 0.5g
[0048] lower level:
[0049] Capsaicin (natural extract) 27.52mg
[0050] Hydroxypropyl-β-cyclodextrin (HP-β-CD) 0.3g
[0051] Laurocaprazine (Az) 0.10g
[0052] Rohm Eudragit E100 (Rohm Germany) 0.4g
[0053] Preparation method: upper layer: take the prescribed amount of menthol and borneol, add OA, PG, dibutylphenol, polyacrylate and an appropriate amount of ethyl acetate, stir evenly, vacuum degas, and apply the upper layer glue on the anti-adhesive layer Cloth into a uniform thin layer with a certain thickness, and bake at 60°C for 30 minutes. Lower layer: Take the prescribed amount of capsaicin and HP-β-CD, add appropriate amount of ethanol to dissolve, and ultrasonically clathrate; take ano...
Embodiment 2
[0062] Prescription (43cm 2 Feeding)
[0063] upper layer:
[0064] Dexketoprofen 0.02g
[0065] Borneol 0.08g
[0066] Menthol 0.10g
[0067] Oleic acid (OA) 0.12g
[0068] 1,2-propanediol (PG) 0.25g
[0069] Propyl Perylate 0.008g
[0070] Polyacrylate (87-8525, American Chemical Starch Company) 0.9g
[0071] lower level:
[0072] Capsaicin (synthetic) 27.52mg
[0073] Hydroxypropyl-β-cyclodextrin (HP-β-CD) 0.3g
[0074] Oleic acid (OA) 0.12g
[0075] Dimethylformamide 0.15g
[0076] ROHM Eudragit RL100 (Germany ROHM company) 0.5g
[0077] Polyvinylpyrrolidone (PVP K30) 0.1g
[0078] Preparation method: upper layer: take the prescription amount of dexketoprofen, menthol, propyl pyritate and borneol, add OA, PG, polyacrylate and an appropriate amount of ethyl acetate, stir evenly, vacuum degas, and put the upper layer of glue Coat the anti-adhesive layer into a uniform thin layer with a certain thickness, and bake at 60°C for 30 minutes. Lower layer: take the pr...
Embodiment 3
[0084] Prescription (43cm 2 Feeding)
[0085] upper layer:
[0086] Flurbiprofen 0.0185g
[0087] borneol 0.15g
[0088] Oleic acid (OA) 0.10g
[0089] 1,2-propanediol (PG) 0.25g
[0090] Dibutylphenol 0.02
[0091] Polyacrylate (87-2677, American Chemical Starch Company) 1.0g
[0092] lower layer:
[0093] Dihydrocapsaicin 21.5mg
[0094] Hydroxypropyl-β-cyclodextrin (HP-β-CD) 0.25g
[0095] Ethyl oleate 0.06g
[0096] Tween-80 0.10g
[0097] Hydroxypropylmethylcellulose (HPMC) 0.06g
[0098] Eudragit L100 (German Rohm company) 0.35g
[0099] Upper layer: Take the prescribed amount of flurbiprofen and borneol, add OA, PG, polyacrylate, dibutylphenol and an appropriate amount of ethyl acetate, stir evenly, vacuum degas, and coat the upper layer of glue on the anti-adhesive layer Form a uniform thin layer with a certain thickness and bake at 60°C for 30 minutes. Lower layer: take the prescribed amount of dihydrocapsaicin and HP-β-CD, add appropriate amount of etha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com