Gel coating accessory capable of preventing spinal surgery cerebrospinal fluid leakage
A surgical operation and coating technology, applied in the field of medical devices, can solve the problems of anesthesia, affecting the effect of hemostasis, and unsatisfactory hemostasis effect, and achieve the effects of preventing infection, promoting wound healing, and good biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Gel Applicator Kit:
[0067] 1) The first fluid component: boric acid buffer solution (pH 6; 5ml) of polylysine (0.1g);
[0068] 2) Second fluid component: phosphate buffer (pH 11; 5ml), in which dexamethasone (0.1g) is dissolved;
[0069]3) Solid component: polytrimethylene carbonate and polylactic acid copolymer (2g; weight average molecular weight 12KD);
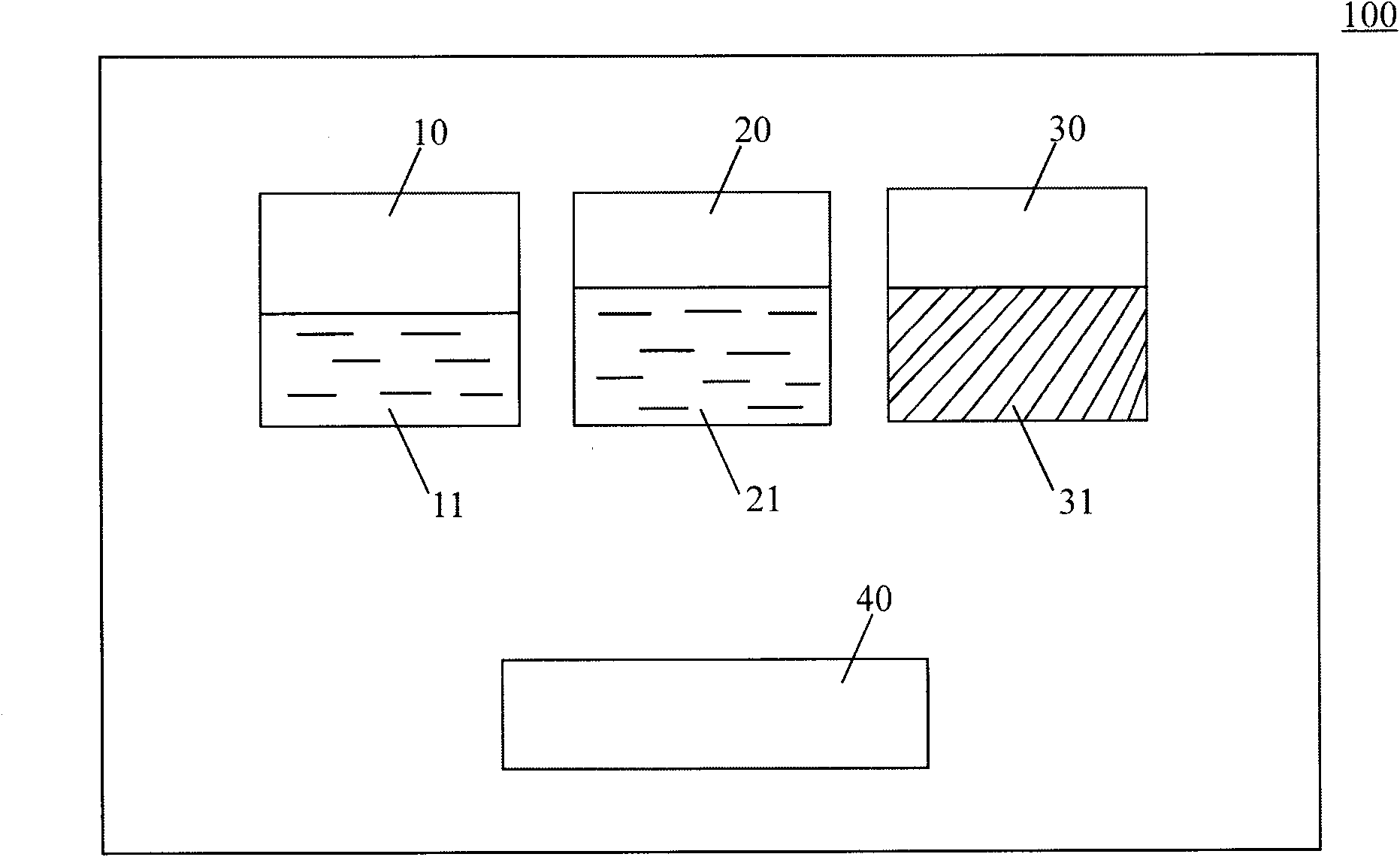
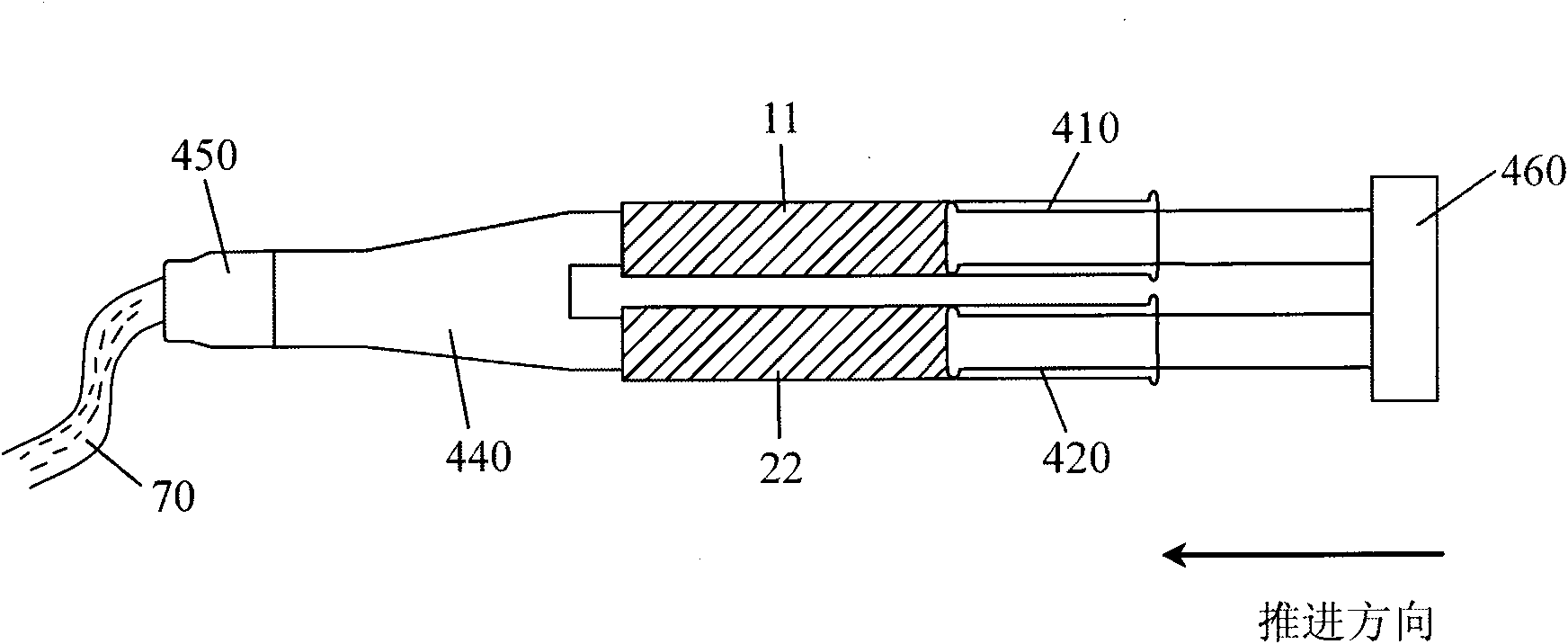
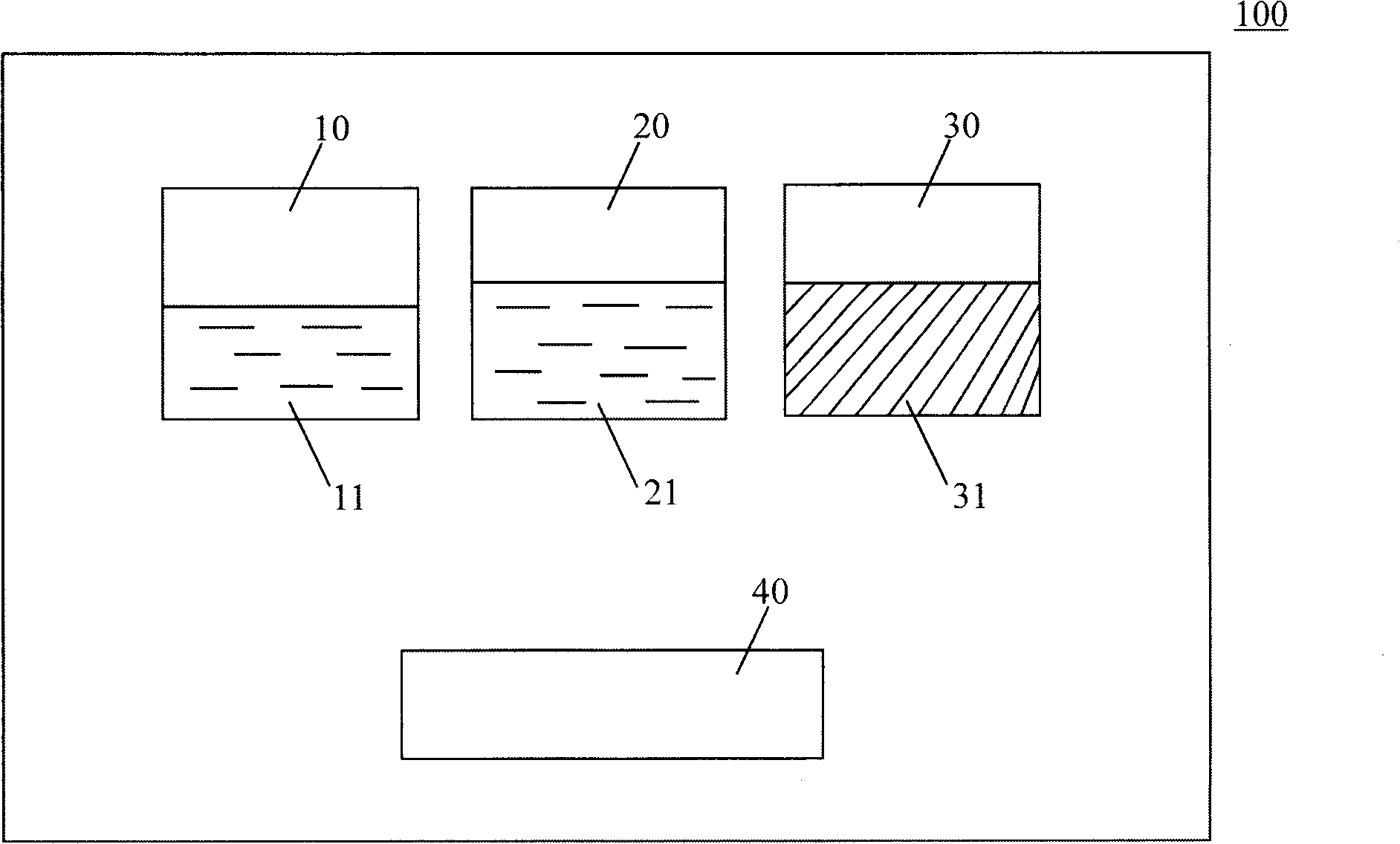
[0070] 4) Coating tools: (such as figure 2 shown).
[0071] Preparation: Dissolve 0.1g polylysine powder in 5ml boric acid buffer solution with a pH value of 6, then dissolve 0.1g dexamethasone powder in 5ml phosphate buffer solution with a pH value of 11, and fill them in In two syringes 410 and 420; weigh 2g of polytrimethylene carbonate and polylactic acid copolymer powder with a weight average molecular weight of 12KD and put it in the third container 30; configure coating tools; seal the package; irradiate sterilization;
[0072] Application: Before use, dissolve the solid component 31 in the third contai...
Embodiment 2
[0074] Gel Applicator Kit:
[0075] 1) The first fluid component: boric acid buffer solution (pH 10; 2ml) of polylysine (0.04g);
[0076] 2) The second fluid component: phosphate buffer (pH 4; 5ml);
[0077] 3) Solid component: polyethylene glycol polybranch isomer (0.7g; weight average molecular weight 30KD);
[0078] 4) Coating tools: (such as figure 2 shown).
[0079] Preparation: Dissolve 0.04g of polylysine powder in 2ml of borate buffer solution with pH value of 10, then fill 5ml of phosphate buffer solution with pH value of 4 into two syringes 410 and 420 respectively; weigh 0.7g polyethylene glycol polybranched isomer powder with a weight average molecular weight of 30KD is packed in a third container 30; coating tools are configured; sealed packaging; irradiation sterilization;
[0080] Application: Before use, dissolve the solid component 31 in the third container 30 into the second fluid component 21 in the syringe 420, and at the same time, install a tee and a...
Embodiment 3
[0082] Gel Applicator Kit:
[0083] 1) The first fluid component: boric acid buffer solution (pH 12; 5ml) of dimerized lysine (0.2g);
[0084] 2) The second fluid component: phosphate buffer (pH 3; 5ml);
[0085] 3) Solid components: gelatin (0.1g) and gelatin protein (1g; weight average molecular weight 30KD);
[0086] 4) Coating tools: (such as figure 2 shown).
[0087] Preparation: Dissolve 0.2g of dimerized lysine powder in 5ml of borate buffer solution with a pH value of 12, then fill 5ml of phosphate buffer solution with a pH value of 3 into two syringes 410 and 420 respectively; weigh 0.1g of white gelatin powder and 1g of gelatin protein powder with a weight average molecular weight of 30KD are packed in the third container 30; coating tools are configured; sealed packaging; irradiation sterilization;
[0088] Application: Before use, the solid component 31 in the third container 30 is dissolved in the second fluid component 21 in the syringe 420, and the front en...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com