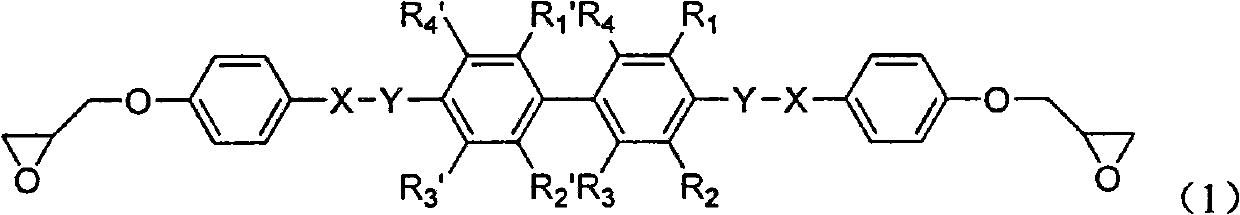
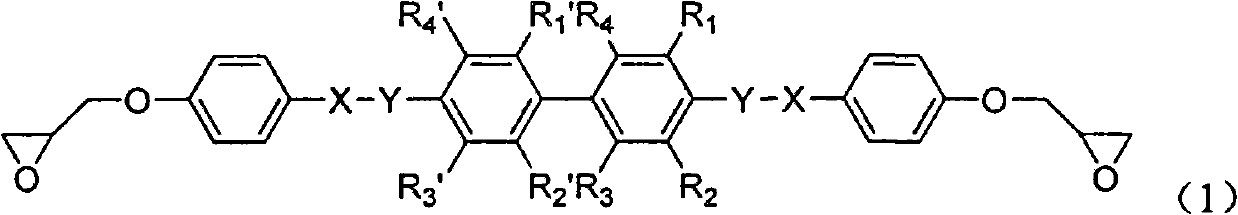
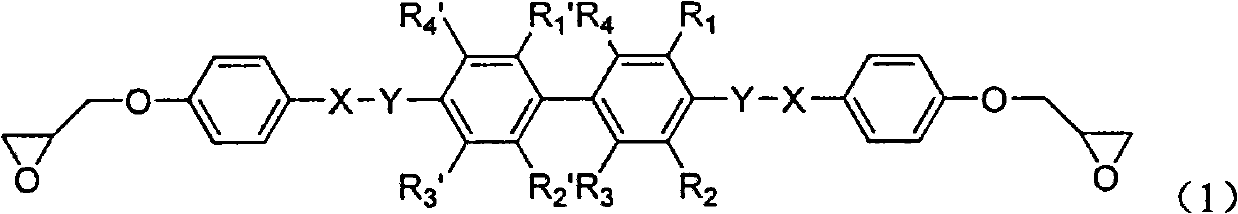
Biphenyl-containing compound liquid crystal epoxy resin and preparation method thereof
An epoxy resin, composite technology, applied in liquid crystal materials, chemical instruments and methods, organic chemistry and other directions, can solve the problems of uneconomical synthesis methods and low yields, improve appearance quality, high reaction conversion rate, prevent The effect of oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Step 1: Synthesis of 2-p-hydroxybenzoic acid-3,3′,5,5′-tetramethoxybiphenyl-4,4′-diester
[0030] Under nitrogen protection, p-hydroxybenzoic acid (0.2mol, 27.6g), 3,3',5,5'-tetramethoxybiphenyl-4,4'-diphenol (0.05mol, 15.3g), p- Toluenesulfonic acid (3mmol, 0.54g) and sulfolane (80ml) were added to a three-necked flask equipped with a stirring bar, water trap and thermometer and stirred at 220°C for 6h. The water formed during the reaction was continuously removed. After the reaction mixture was cooled to room temperature, it was poured into 500 ml of water, and the filter cake obtained after filtration was repeatedly washed with water and ethanol. Dip-hydroxybenzoic acid-3,3',5,5'-tetramethoxybiphenyl-4,4'-diester (23.3 g) was finally obtained as a white powder. Yield: 85.4%.
[0031] Step 2: Synthesis of bis(4-(2,3-epoxypropoxy)benzoic acid)-3,3',5,5'-tetramethoxybiphenyl-4,4'-diester
[0032] Under nitrogen protection, di-p-hydroxybenzoic acid-3,3',5,5'-tetramet...
Embodiment 2
[0037] The synthetic method of the present embodiment is:
[0038]
[0039] Step 1: Synthesis of 2-p-hydroxybenzoic acid-3,3′,5,5′-tetramethylbiphenyl-4,4′-diester
[0040] Under nitrogen protection, p-hydroxybenzoic acid (0.2mol, 27.6g), 3,3', 5,5'-tetramethylbiphenyldiphenol (0.05mol, 12.1g), p-toluenesulfonic acid (5mmol , 0.9g) and sulfolane (50ml) were added to a three-necked flask with a stirring bar, a water trap and a thermometer and stirred at 180°C for 4h. The water formed during the reaction was continuously removed. After the reaction mixture was cooled to room temperature, it was poured into 500 ml of water, and the filter cake obtained after filtration was repeatedly washed with water and ethanol. Dip-hydroxybenzoic acid-3,3',5,5'-tetramethylbiphenyl-4,4'-diester (19.3 g) was finally obtained as a white powder. Yield: 80.1%.
[0041] Step 2: Synthesis of bis(4-(2,3-epoxypropoxy)benzoic acid)-3,3',5,5'-tetramethylbiphenyl-4,4'-diester
[0042] Under nitrog...
Embodiment 3
[0045] The synthetic method of the present embodiment is:
[0046]
[0047] image 3
[0048] Step 1: N,N'-bis(4-hydroxyphenylmethylene)-2,2',3,3',5,5',6,6'-octafluorobiphenyl-4,4'- Synthesis of diamines
[0049] Under nitrogen protection, p-hydroxybenzaldehyde (0.2mol, 24.4g), 2,2',3,3',5,5',6,6'-octafluorobiphenyl-4,4'-diamine ( 0.05mol, 16.4g) and dimethylsulfoxide (40ml) were added to a three-necked flask with a stirring bar and a thermometer and stirred at 100°C for 3h. After the reaction mixture was cooled to room temperature, it was poured into 500 ml of water, and the filter cake obtained after filtration was repeatedly washed with water and ethanol. Finally, N,N'-bis(4-hydroxyphenylmethylene)-2,2',3,3',5,5',6,6'-octafluorobiphenyl-4 was obtained as pink powder , 4'-diamine (25.5 g). Yield: 95.2%.
[0050] Step 2: N,N'-bis(4-(2,3-epoxypropoxy)-phenylmethylene)-2,2',3,3',5,5',6,6' -Synthesis of octafluorobiphenyl-4,4'-diamine
[0051] Under nitrogen protection...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com