Preparation method of high-content sesquiterpenoids tripterygium alkaloid
A technology of Tripterygium wilfordii alkaloids and sesquiterpenoids, which is applied in the field of medicine, can solve the problems of unfavorable pharmacological research of tripterygium wilfordii sesquiterpenoids alkaloids and difficult to meet the purity, and achieve the effect of low cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
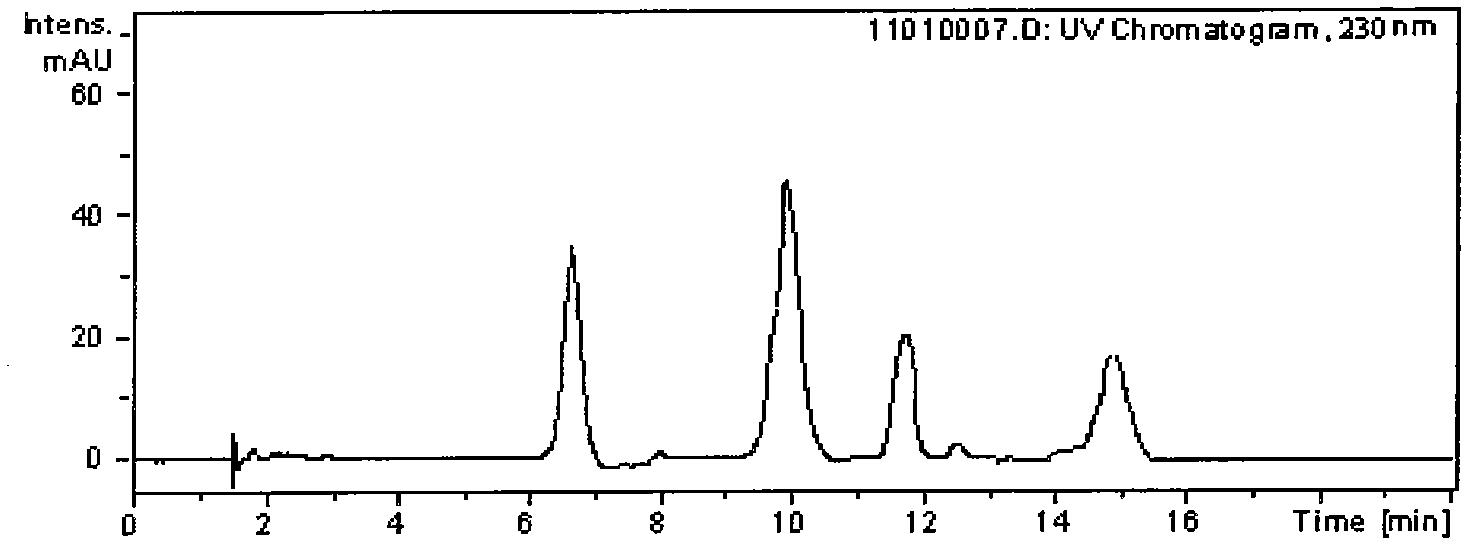
[0024] Take 5.0g of triptolide and triptolide ethyl acetate extract waste liquid from which triptolide and triptolide have been removed, evaporate the solvent, weigh 2.0g in a beaker, extract with 10mL chloroform each time, repeat 3 times , combined the chloroform layers, and evaporated to dry to obtain the chloroform extract; then the chloroform extract was extracted 3 times with 10g / L acetic acid, the acetic acid extract was combined, and the pH was adjusted to 10 with 10g / L ammonia water, and the precipitate was filtered with deionized Wash with water for 5 times and dry to obtain crude total alkaloids, then add 30.0 mL of methanol to dissolve, carry out recrystallization at room temperature, and use the raw materials obtained by recrystallization for chromatographic separation.
[0025] The crude total alkaloids of Tripterygium wilfordii obtained by recrystallization were dissolved in a mixed solvent of acetonitrile and water with a volume ratio of 60:40, and then a mixture...
Embodiment 2
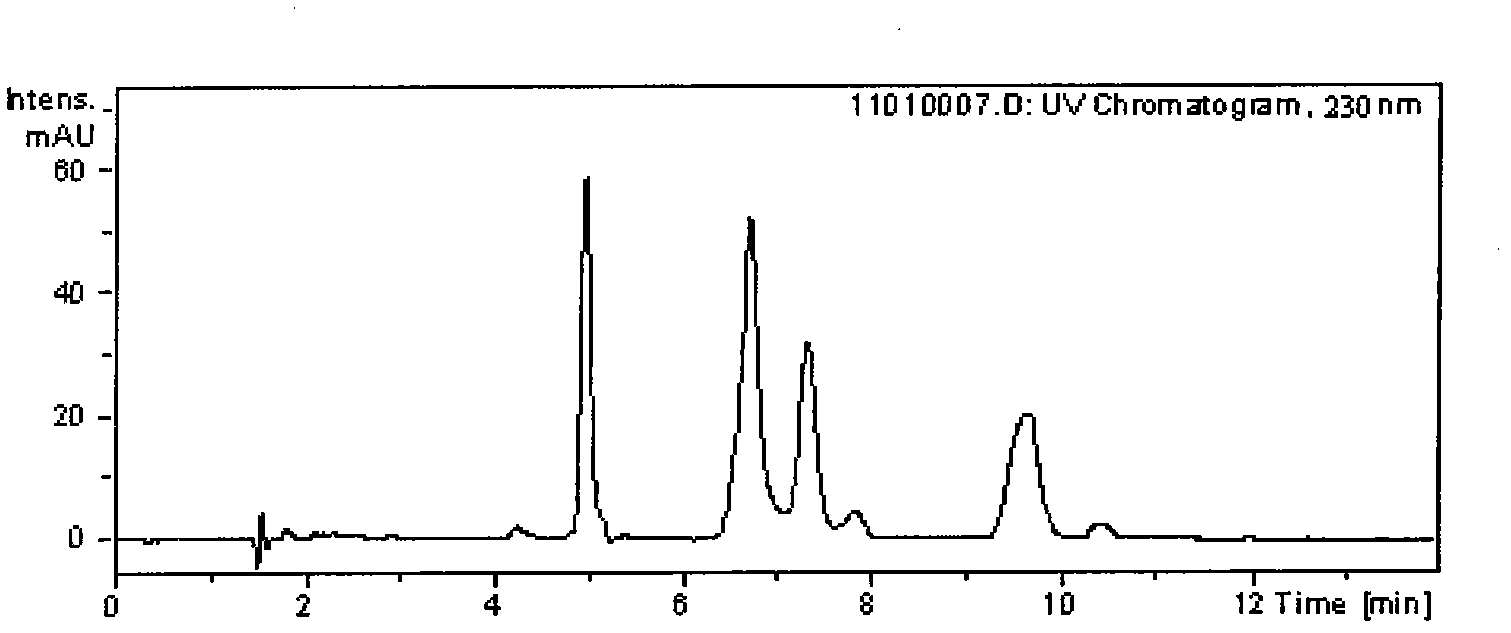
[0027] Take 5.0g of triptolide and triptolide ethyl acetate extract waste liquid from which triptolide and triptolide have been removed, evaporate the solvent, weigh 2.0g in a beaker, extract with 10mL chloroform each time, repeat 3 times , combined the chloroform layers, and evaporated to dry to obtain the chloroform extract; then the chloroform extract was extracted 3 times with 10g / L formic acid, the formic acid extract was combined, and the pH was adjusted to 6 with 15g / L formic acid ammonia water, and the precipitate was filtered and used Wash 5 times with deionized water and dry to obtain crude total alkaloids, then add 20.0 mL of isopropanol to dissolve, carry out recrystallization at room temperature, and use the raw materials obtained by recrystallization for chromatographic separation.
[0028] The crude total alkaloids of Tripterygium wilfordii obtained by recrystallization were dissolved in a mixed solvent of acetonitrile and water with a volume ratio of 99:1, and t...
Embodiment 3
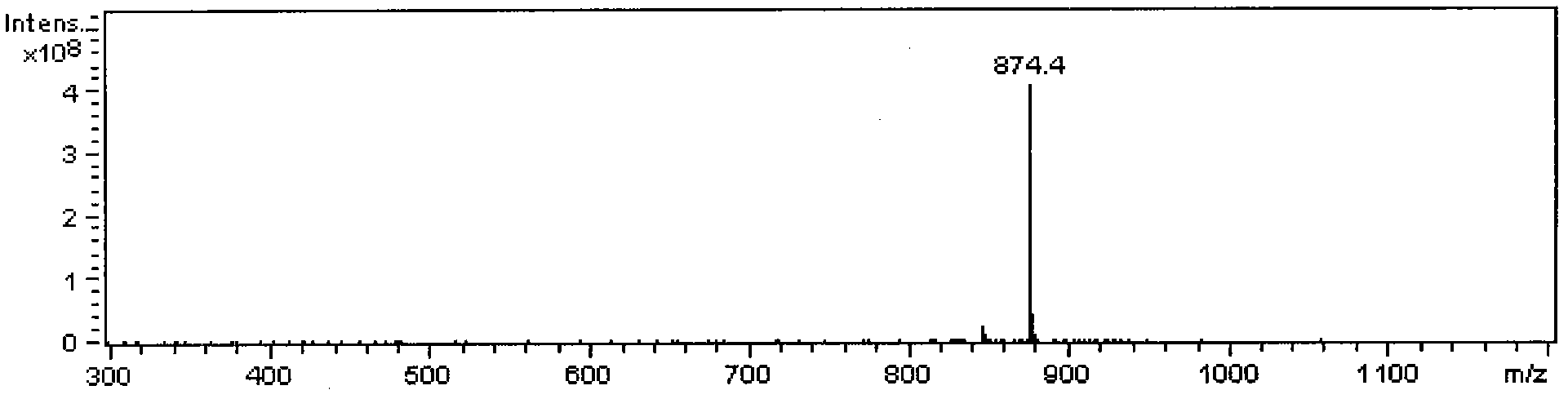
[0030] Take 10.0g of triptolide and triptolide ethyl acetate extract waste liquid from which triptolide and triptolide have been removed, evaporate the solvent, weigh 2.0g in a beaker, extract with 10mL chloroform each time, repeat 3 times , combined the chloroform layers, and evaporated to dry to obtain the chloroform extract; then the chloroform extract was extracted 3 times with 10g / L acetic acid, the acetic acid extract was combined, and the pH was adjusted to 10 with 8g / L ammonia water, and the precipitate was filtered with deionized Wash with water for 5 times and dry to obtain crude total alkaloids, then add 10.0-50.0mL of methanol to dissolve, carry out recrystallization at room temperature, and use the raw materials obtained by recrystallization for chromatographic separation.
[0031] The crude total alkaloids of Tripterygium wilfordii obtained by recrystallization are dissolved in a mixed solvent of acetonitrile and water with a volume ratio of 75:25, and the mixture...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com