Leptospira interrogans DNA (Deoxyribose Nucleic Acid) vaccine as well as construction method and application thereof
A leptospira and DNA vaccine technology, which is applied in the field of medicine and biology, can solve the problems such as no reports of Leptospira question mark DNA vaccine, and achieves the effects of stable physical and chemical properties, convenient storage and transportation, and simple production process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: The structure, construction and preparation of the DNA vaccine pVAX-LB018 of the present invention:
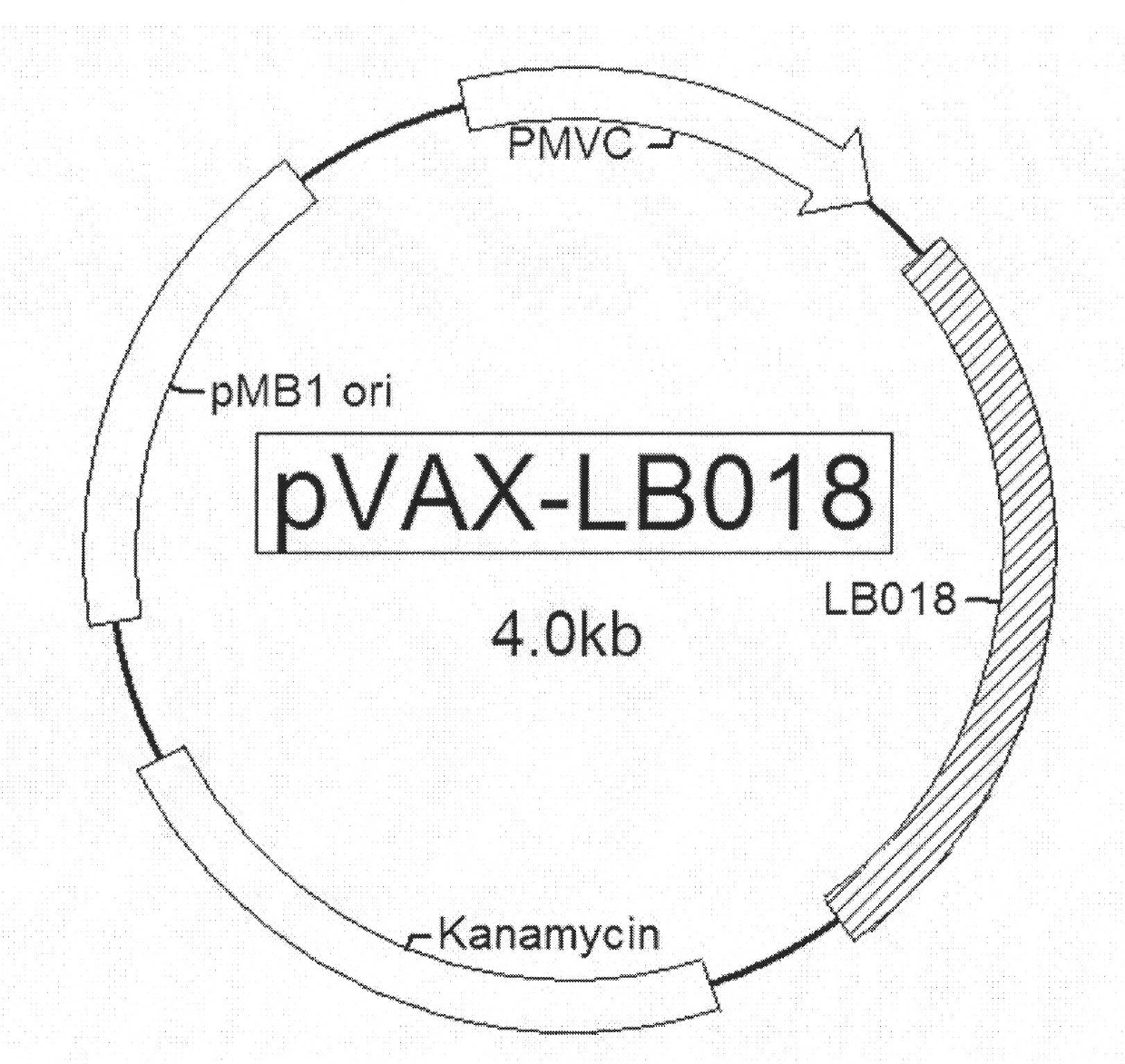
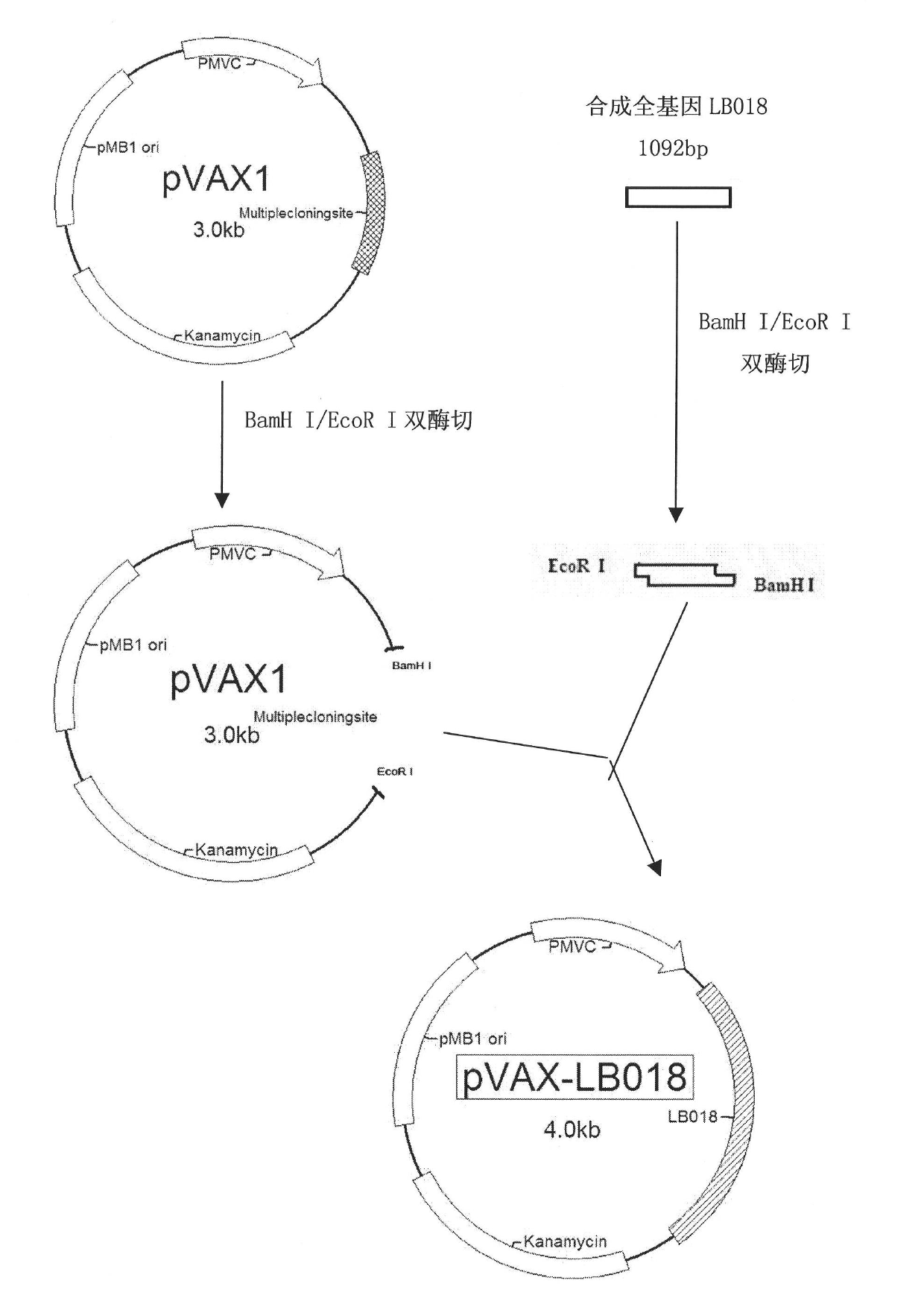
[0044] The structure of the DNA vaccine of the present invention is a recombinant plasmid DNA molecule (purchased from Invitrogen Company) with the eukaryotic expression vector—pVAX as the backbone, and the general structure is as follows: figure 1 shown. The total length is about 4.0kb (kilobase pairs), and besides containing the pMB1 ori plasmid replication initiation site, kanamycin resistance gene Kanamycin and other elements, it also contains a complete eukaryotic transcription and translation unit. The eukaryotic transcription-translation unit contains elements necessary for eukaryotic transcription and translation: eukaryotic cytomegalovirus promoter P CMV , the corresponding coding gene LB018 and the polyadenylic acid BGH pA at the 3' end. Generally speaking, the vaccine is a recombinant plasmid DNA that can express LB018 in eukaryotic cells and...
Embodiment 2
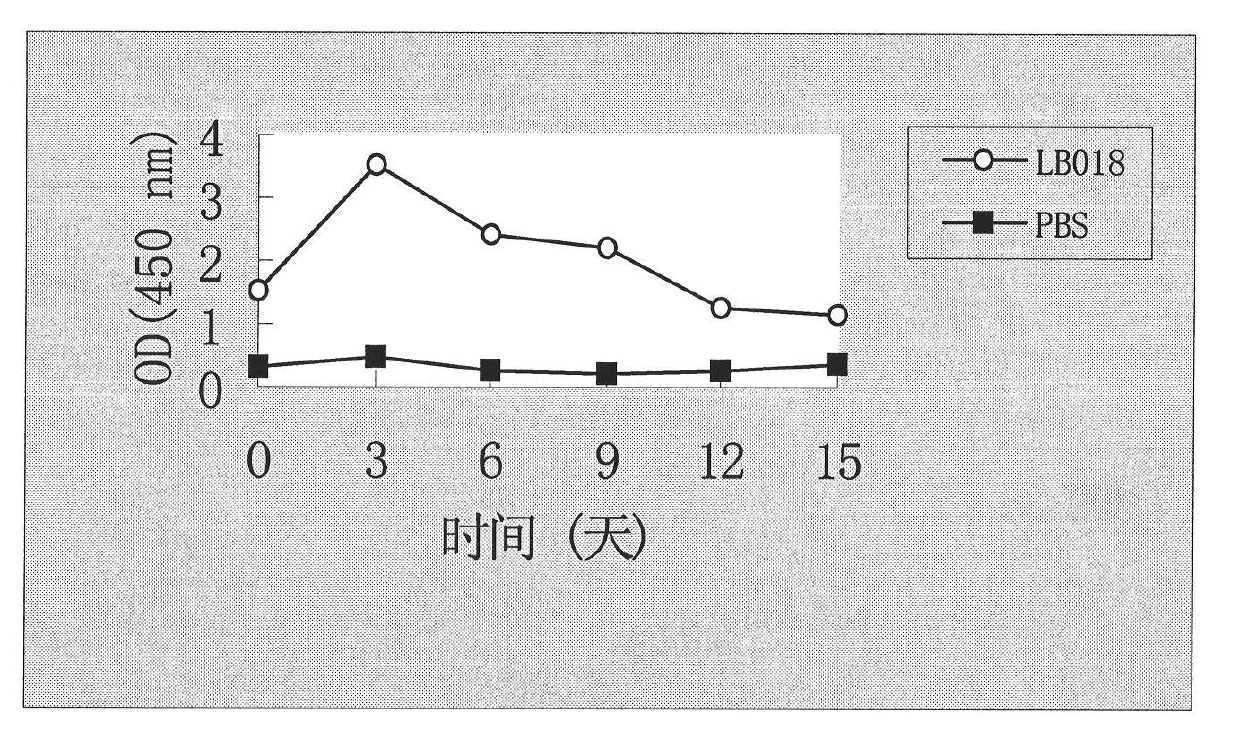
[0054] Example 2: Functional testing of the DNA vaccine pVAX-LB018 of the present invention:
[0055] 1, obtain the DNA vaccine of the present invention of high purity:
[0056] Take out the frozen engineered strain——DH5α(pVAX-LB018) from the -80°C refrigerator, and after thawing at room temperature, use an inoculation loop to pick up a small amount of bacterial solution and inoculate it on a 1.5% LB agar plate (containing kanamycin). 50ug / ml), after culturing overnight at 37°C, take a single clone and inoculate it in 50ml LB liquid medium (containing 1% tryptone, 0.5% yeast extract and 1% sodium chloride, kanamycin 50ug / ml, pH7 .0), cultured with shaking at 37°C for 40 hours, and used as seed solution. The seed solution was inoculated in 1 liter of SOC liquid medium (on the basis of the above-mentioned components, additionally containing 50 μg / ml kanamycin) at a ratio of 1:20, and cultured with vigorous shaking at 37°C for 25 hours; To a final concentration of 170 μg / ml, cu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com