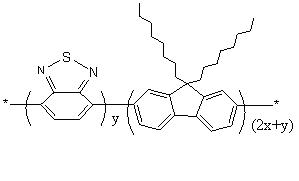
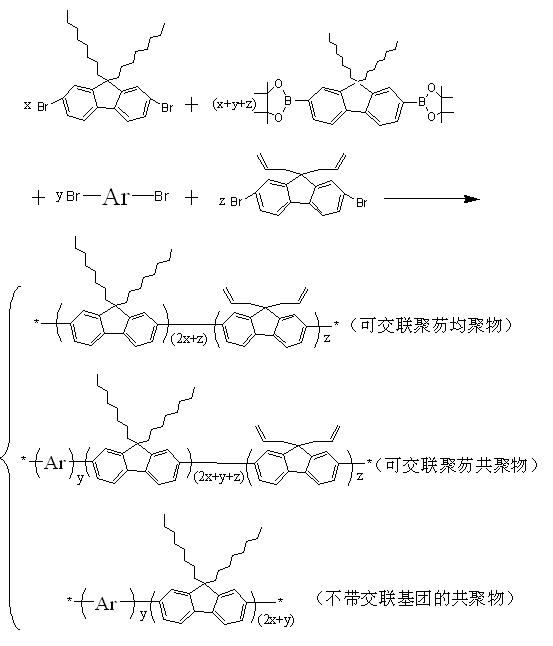Fluorene polymer with crosslinkable group, crosslinked film and preparation method thereof
A technology of cross-linking groups and polymers, which is applied in the field of cross-linked films and their preparation, can solve the problems of complex preparation and low cross-linking degree, and achieve the effects of simple preparation, fast cross-linking speed and convenient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: (synthesis of a homopolymer whose molar ratio of crosslinkable units is 50% fluorene)
[0049] Polymerization recipe:
[0050] Reactant II (2,7-dibromo-9,9-diallylfluorene) and reactant III (2,7-bis(4,4,5,5-tetramethyl-1,3,2- Dioxaborane-diyl)-9,9-dioctylfluorene) in a molar ratio of 1:1;
[0051] The molar ratio of catalyst and reactant III is 0.03:1;
[0052] The molar ratio of amine ligand to catalyst is 12:1;
[0053]The mol ratio of alkali and reactant III is 6:1;
[0054] Aggregation steps:
[0055] 0.321 g (0.5 mmol) of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-diyl)-9,9-dioctylfluorene ( III), 0.202g (0.25mmol) 2,7-dibromo-9,9-diallyl fluorene (II), 0.0034g (0.015mmol) palladium catalyst Pd(OAc) 2 , 0.0202g (0.18mmol) ligand triethylenediamine, 0.0249g (0.18mmol) potassium carbonate, and 5ml toluene were added successively in a clean 100ml three-necked flask equipped with magnets, vacuumed and ventilated nitrogen, and the temperature was ...
Embodiment 2
[0056] Example two: (synthesis of a homopolymer whose molar ratio of crosslinkable units is 25% fluorene)
[0057] Polymerization recipe:
[0058] Reactant I (2,7-dibromo-9,9-dioctylfluorene), Reactant II (2,7-dibromo-9,9-diallylfluorene), Reactant III (2,7 - bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-diyl)-9,9-dioctylfluorene) in a molar ratio of 1:1:2;
[0059] The molar ratio of catalyst and reactant III is 0.03:1;
[0060] The molar ratio of amine ligand to catalyst is 12:1;
[0061] The mol ratio of alkali and reactant III is 6:1;
[0062] Aggregation steps:
[0063] 0.321 g (0.5 mmol) of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-diyl)-9,9-dioctylfluorene ( III), 0.137g (0.25mmol) 2,7-dibromo-9,9-dioctylfluorene (I), 0.101g (0.25mmol) 2,7-dibromo-9,9-diallylfluorene (II), 0.0034g (0.015mmol) palladium catalyst Pd(OAc) 2 , 0.0202g (0.18mmol) of ligand triethylenediamine, 0.415g (3mmol) of potassium carbonate, and 5ml of toluene were added successively in a c...
Embodiment 3
[0064] Example three: (synthesis of a homopolymer whose molar ratio of crosslinkable units is 20% fluorene)
[0065] Polymerization recipe:
[0066] Reactant I (2,7-dibromo-9,9-dioctylfluorene), Reactant II (2,7-dibromo-9,9-diallylfluorene), Reactant III (2,7 - bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-diyl)-9,9-dioctylfluorene) in a molar ratio of 3:2:5
[0067] The molar ratio of catalyst to reactant III is 0.03:1
[0068] The molar ratio of amine ligand to catalyst is 12:1
[0069] The molar ratio of base to reactant III is 6:1
[0070] Aggregation steps:
[0071] 0.321 g (0.5 mmol) of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-diyl)-9,9-dioctylfluorene ( III), 0.164g (0.30mmol) 2,7-dibromo-9,9-dioctylfluorene (I), 0.0808g (0.20mmol) 2,7-dibromo-9,9-diallylfluorene (II), 0.0034g (0.015mmol) palladium catalyst Pd(OAc) 2 , 0.0202g (0.18mmol) of ligand triethylenediamine, 0.415g (3mmol) of potassium carbonate, and 5ml of toluene were added successively in a clea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com