Nonaqueous electrolyte, and nonaqueous electrolyte secondary battery using same
A non-aqueous electrolyte, secondary battery technology, applied in non-aqueous electrolyte storage batteries, secondary batteries, battery electrodes, etc., can solve the problem of increased battery gas generation, non-aqueous electrolyte difficult to penetrate the electrode plate, easy to be oxidized and decomposed, etc. problem, to achieve the effect of excellent low temperature characteristics, excellent storage characteristics, and excellent charge-discharge cycle characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
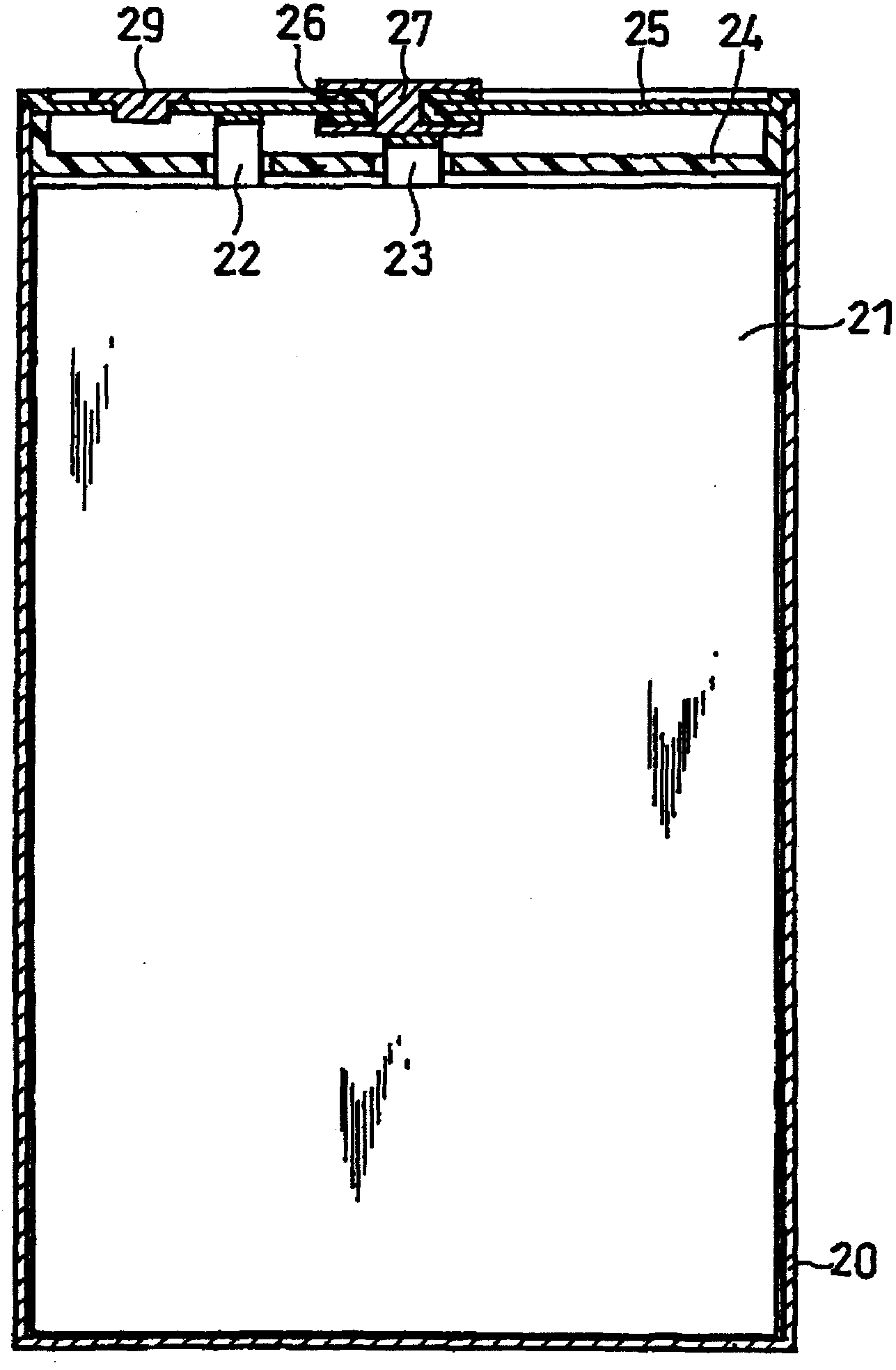
Image
Examples
Embodiment 1
[0099] (a) Preparation of negative electrode
[0100] Process (i)
[0101] First, carboxymethylcellulose (hereinafter referred to as CMC, molecular weight: 400,000), which is a water-soluble polymer, was dissolved in water to obtain an aqueous solution having a CMC concentration of 1.0% by weight. Mix 100 parts by weight of natural graphite particles (the average particle diameter is 20 μm, the average circularity is 0.92, and the specific surface area is 4.2m 2 / g) and 100 parts by weight of the CMC aqueous solution, while controlling the temperature of the mixture to 25°C, it was stirred. Thereafter, the mixture was dried at 120° C. for 5 hours to obtain a dry mixture. In the dry mixture, the amount of CMC was 1.0 parts by weight per 100 parts by weight of graphite particles.
[0102] Process (ii)
[0103] 101 parts by weight of the obtained dry mixture, 0.6 parts by weight, 0.9 parts by weight of a particle-shaped, styrene unit and butadiene unit-containing, rubber-elas...
Embodiment 2
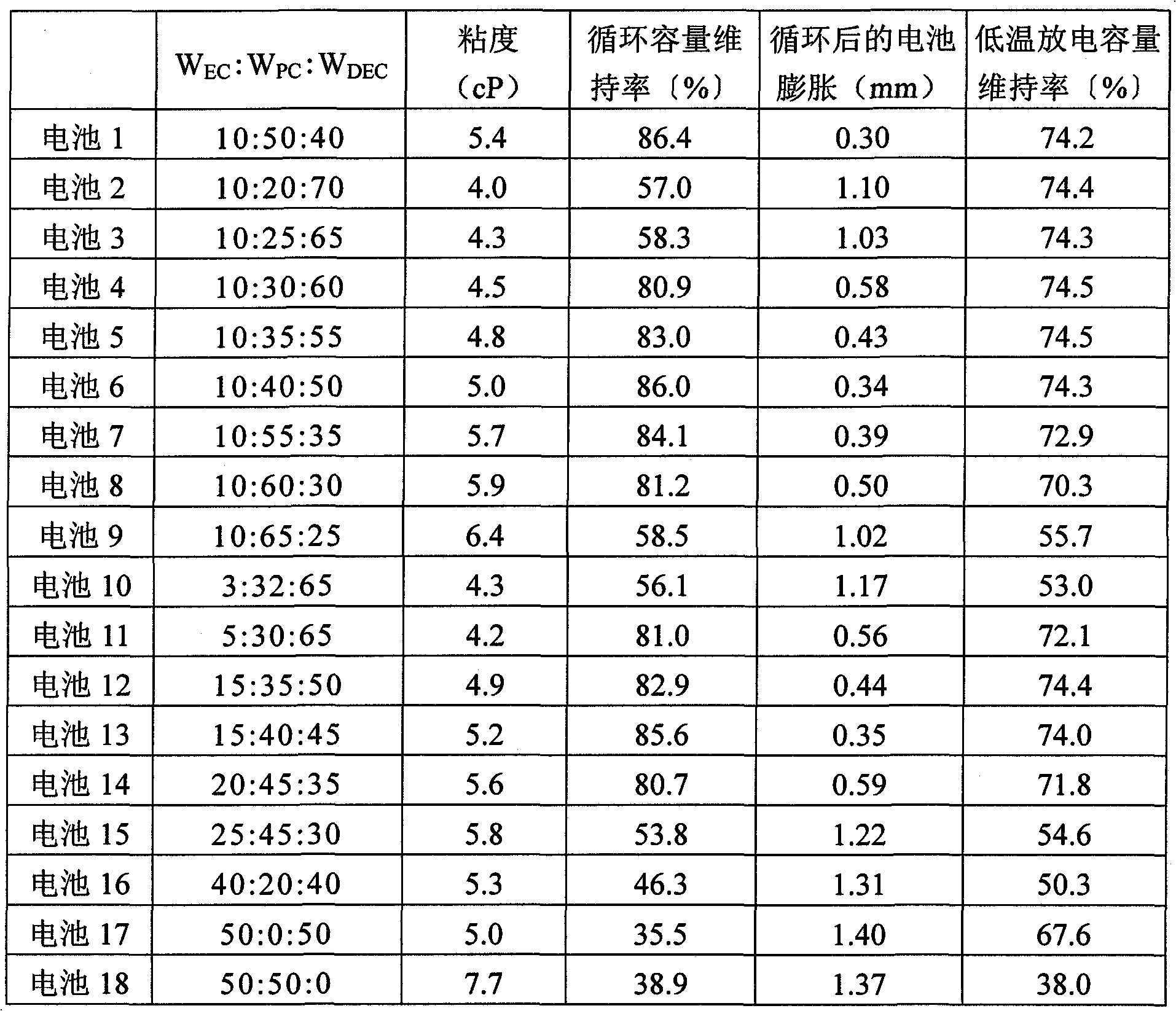
[0131] In addition to using W EC :W PC :W DEC A nonaqueous electrolyte was prepared in the same manner as in Example 1 except that it was changed as in Table 1. Batteries 2 to 18 were produced in the same manner as in Example 1 except that the obtained nonaqueous electrolyte was used. In addition, Batteries 2, 3, 9, 10, and 15 to 18 are all batteries of comparative examples.
[0132] Batteries 2 to 18 were evaluated in the same manner as in Example 1. The results are shown in Table 1.
[0133] Table 1
[0134]
[0135] It can be seen from Table 1 that for the weight ratio W of PC PC 30-60% by weight, W C / W SLThe battery with a non-aqueous electrolyte of 1.0 has good cycle capacity retention rate and low-temperature discharge capacity retention rate. Furthermore, it can be seen that the battery after the cycle has little swelling and the amount of gas generated is also small. Furthermore, for the weight ratio W using PC PC and the weight ratio of EC W EC Ratio: ...
Embodiment 3
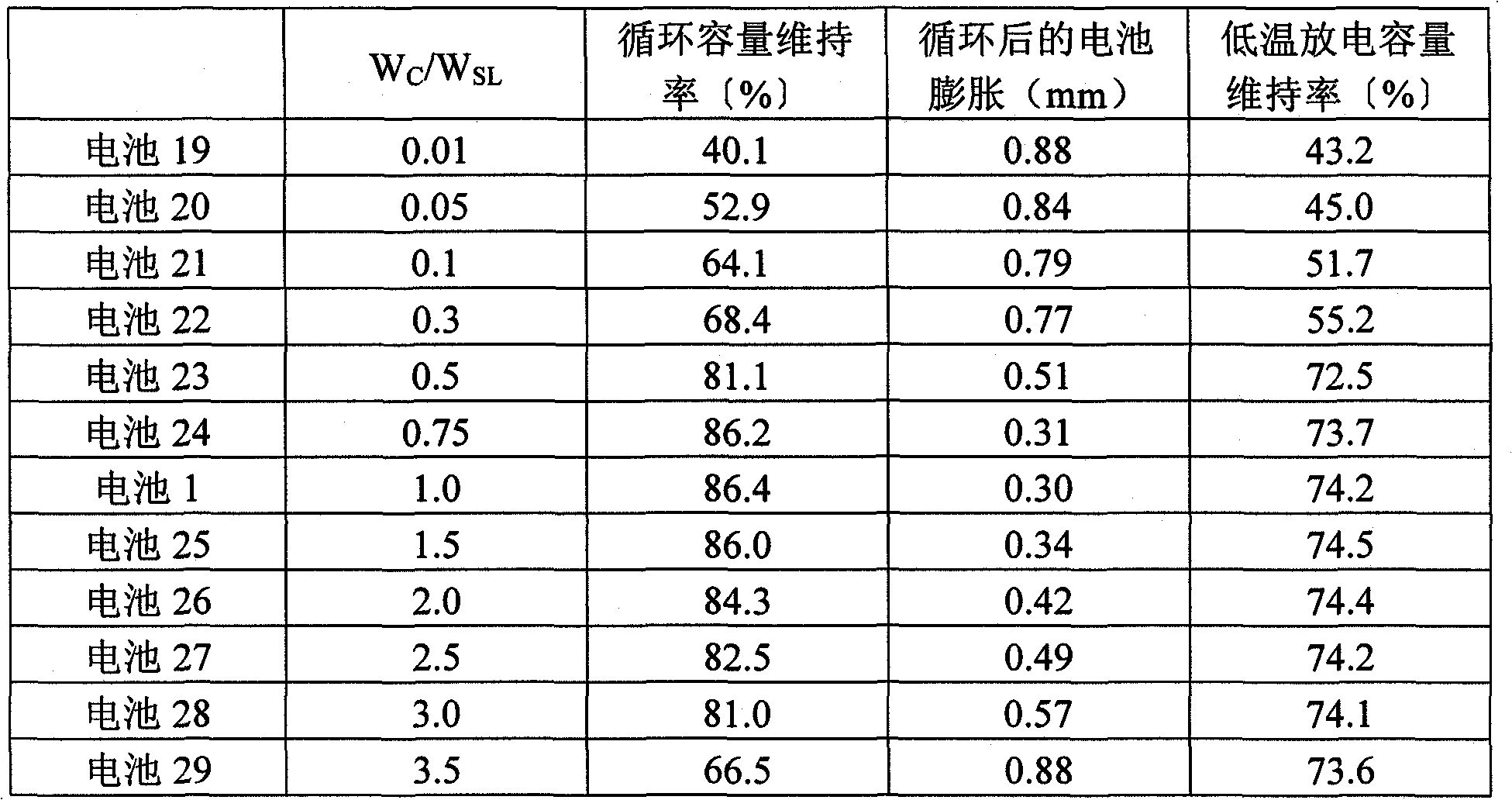
[0141] The total amount except additives is set to 3.0% by weight, so that W C / W SL A nonaqueous electrolyte was prepared in the same manner as in Example 1 except that it was changed as in Table 2. Batteries 19 to 29 were fabricated in the same manner as in Example 1 except that the obtained nonaqueous electrolyte was used. In addition, batteries 19 to 22 and battery 29 are batteries of comparative examples.
[0142] Batteries 19 to 29 were evaluated in the same manner as in Example 1. The results are shown in Table 2.
[0143] Table 2
[0144]
[0145] As can be seen from Table 2, for the use of the weight ratio W of cyclic carbonate (VC) with C=C unsaturated bond C The weight ratio W with the sultone compound (PS) SL Ratio: W C / W SL Satisfy 0.5≤W C / W SL The non-aqueous electrolyte batteries with ≤3.0 have particularly good cycle capacity retention rate and low-temperature discharge capacity retention rate. In addition, the battery swells after cycling with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com