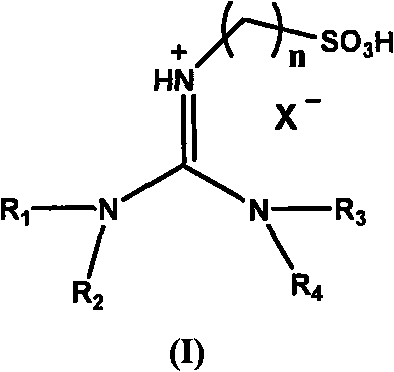
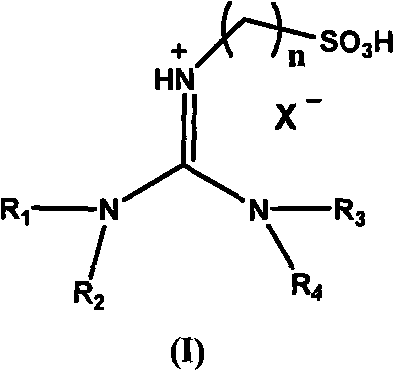
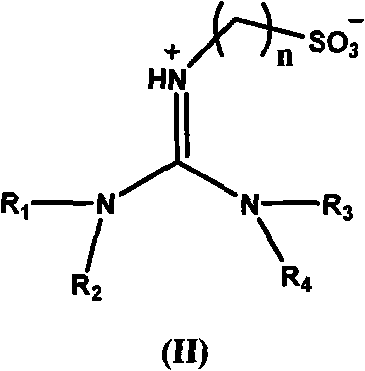
Acidic room-temperature ionic liquid using acidic functional tetraalkylguanidine as cations and preparation method thereof
A technology of room temperature ionic liquid and tetraalkylguanidine, which is applied in the field of acidic room temperature ionic liquid and its preparation, can solve the problems of easy coke deactivation, poor thermal stability, environmental pollution, etc., and achieves environmental protection, easy separation, no Effects of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: 1,1,3, the synthesis of 3-tetramethyl-2-propanesulfonate guanidine bisulfate ion liquid
[0035] A, preparation 1,1,3,3-tetramethyl-2-propanesulfonate root guanidinium salt
[0036] 1,1,3,3-Tetramethylguanidine (0.3mol) and 1,3-propane sultone (0.3mol) were stirred and reacted for 4 hours at 80-85°C in toluene, and the resulting white solid was washed with toluene After washing three times and vacuum drying, 1,1,3,3-tetramethyl-2-propanesulfonate guanidinium salt was obtained with a yield of 99.7%.
[0037] B. Preparation of 1,1,3,3-tetramethyl-2-propanesulfonate guanidine bisulfate ionic liquid
[0038] 1,1,3,3-Tetramethyl-2-propanesulfonate guanidinium salt (0.1mol) and concentrated sulfuric acid (0.11mol) in toluene, stirred and reacted at 80°C for 4 hours, the reaction solution was divided into upper and lower layers , remove the toluene of the upper layer and unreacted raw materials, wash the lower layer with toluene, and vacuum-dry for 24 hours to ...
Embodiment 2
[0039] Example 2: Synthesis of 1,1,3,3-tetramethyl-2-propanesulfonate guanidine dihydrogen phosphate ionic liquid
[0040] 1,1,3,3-tetramethyl-2-propanesulfonate guanidinium salt (0.1mol) prepared according to Example 1 and 85% phosphoric acid (0.11mol) were in toluene, stirred and reacted for 4 hours at 80°C , the reaction solution is divided into upper and lower layers, remove the toluene and unreacted raw materials in the upper layer, wash the lower layer with toluene, and dry in vacuum for 24 hours to obtain 1,1,3,3-tetramethyl-2-propanesulfonic acid guanidine Dihydrogen phosphate ionic liquid, yield 90.4%, light brown viscous liquid at room temperature.
Embodiment 3
[0041] Example 3: Synthesis of 1,1,3,3-tetramethyl-2-propanesulfonate guanidine nitrate ionic liquid
[0042] 1,1,3,3-tetramethyl-2-propanesulfonate guanidinium salt (0.1mol) prepared according to Example 1 and 65% concentrated nitric acid (0.11mol) in toluene, stirring reaction at 80°C 4 hours, the reaction solution was divided into upper and lower layers, the upper layer of toluene and unreacted raw materials were removed, the lower layer was washed with toluene, and vacuum-dried for 24 hours to obtain 1,1,3,3-tetramethyl-2-propanesulfonic acid Guanidine nitrate ionic liquid, yield 98.2%, light brown viscous liquid at room temperature.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com