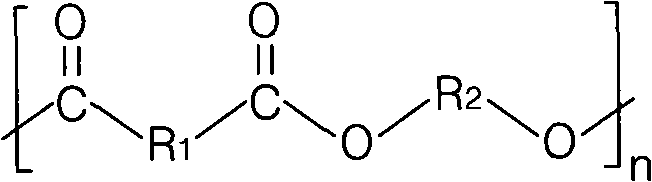
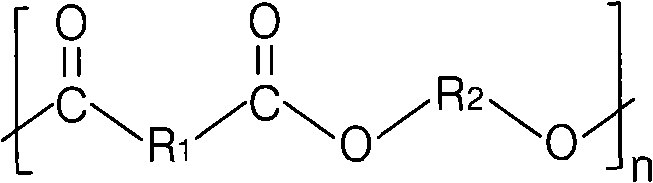
Aromatic-aliphatic block copolyester and preparation method thereof
A block copolyester, aromatic technology, applied in the aromatic-aliphatic block copolyester, the field of preparation of the block copolyester, can solve the problem of crystallinity, crystallization speed decline, can not reach zero interfacial tension , frequent sequence rearrangement and other problems, to achieve the effect of clear sequence structure, easy synthesis and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Step 1. The ring-opening polymerization of cyclic polybutylene terephthalate (CBT), that is, the preparation of macrocyclic polybutylene terephthalate (PBT) containing a catalyst.
[0024] Under nitrogen protection, put 10 g of CBT and 0.5 g of stannoxane catalyst into a 100 ml reaction flask that has been roasted and cooled with nitrogen protection. Anhydrous and anaerobic treated ortho-dichlorobenzene was added with a syringe so that the concentration of the reaction monomer CBT was 2Kg / L. The reaction was carried out at 180°C, and after 10 minutes the reaction was over (GPC monitoring showed no signal peak of CBT), cooled and filtered, and the reaction product was washed with anhydrous chloroform to wash away traces of unreacted CBT monomer, and baked at 60°C Dry.
[0025] Step 2. Catalyst-containing macrocyclic PBT initiates lactide polymerization. The 100ml reaction bottle was also treated anhydrous and oxygen-free, cooled under a nitrogen atmosphere, and then un...
Embodiment 2
[0035] Step 1: Ring-opening polymerization of cyclic polyethylene terephthalate (CET), that is, preparation of catalyst-containing macrocyclic polyethylene terephthalate (PET).
[0036] Under nitrogen protection, put 10 g of CET and 0.05 g of stannoxane catalyst into a 100 ml reaction flask that has been roasted and cooled with nitrogen protection. Anhydrous and anaerobic treated chlorobenzene was added with a syringe so that the concentration of the reaction monomer was 1Kg / L. The reaction was carried out at 120°C. After 48 hours, the reaction was completed (GPC monitoring had no signal peak of CET), cooled and filtered, and the reaction product was washed with chloroform also treated with anhydrous to wash away traces of unreacted CET monomer, and then vacuumed at 60°C drying.
[0037] Step 2. Catalyst-containing macrocyclic PET initiates lactide polymerization. The 25ml reaction bottle was also treated anhydrous and oxygen-free, cooled under a nitrogen atmosphere, and the...
Embodiment 3
[0041] Step 1. The ring-opening polymerization of cyclic polybutylene isophthalate, that is, the preparation of macrocyclic polybutylene isophthalate containing a catalyst.
[0042] Under nitrogen protection, 10 g of cyclic polybutylene isophthalate and 0.1 g of stannoxane catalyst were put into a 50 ml reaction flask that had been roasted and cooled with nitrogen protection. Add a mixed solvent of o-dichlorobenzene and chlorobenzene with a volume ratio of 2:1 after anhydrous and oxygen-free treatment with a syringe, so that the concentration of the reaction monomer cyclic polybutylene isophthalate is 2Kg / L. The reaction was carried out at 180°C, and after 10 minutes the reaction was over (GPC monitored the signal peak of acyclic polybutylene isophthalate), cooled and filtered, and the reaction product was washed with chloroform also treated with anhydrous to wash away traces of Unreacted cyclic monomers were vacuum-dried at 60°C.
[0043] Step 2. Catalyst-containing macrocyc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com