Pharmaceutical formulation containing angiotensin-II receptor blocker
An angiotensin, receptor blocker technology, applied in the field of pharmaceutical preparations, can solve the problem of lack of antioxidants and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
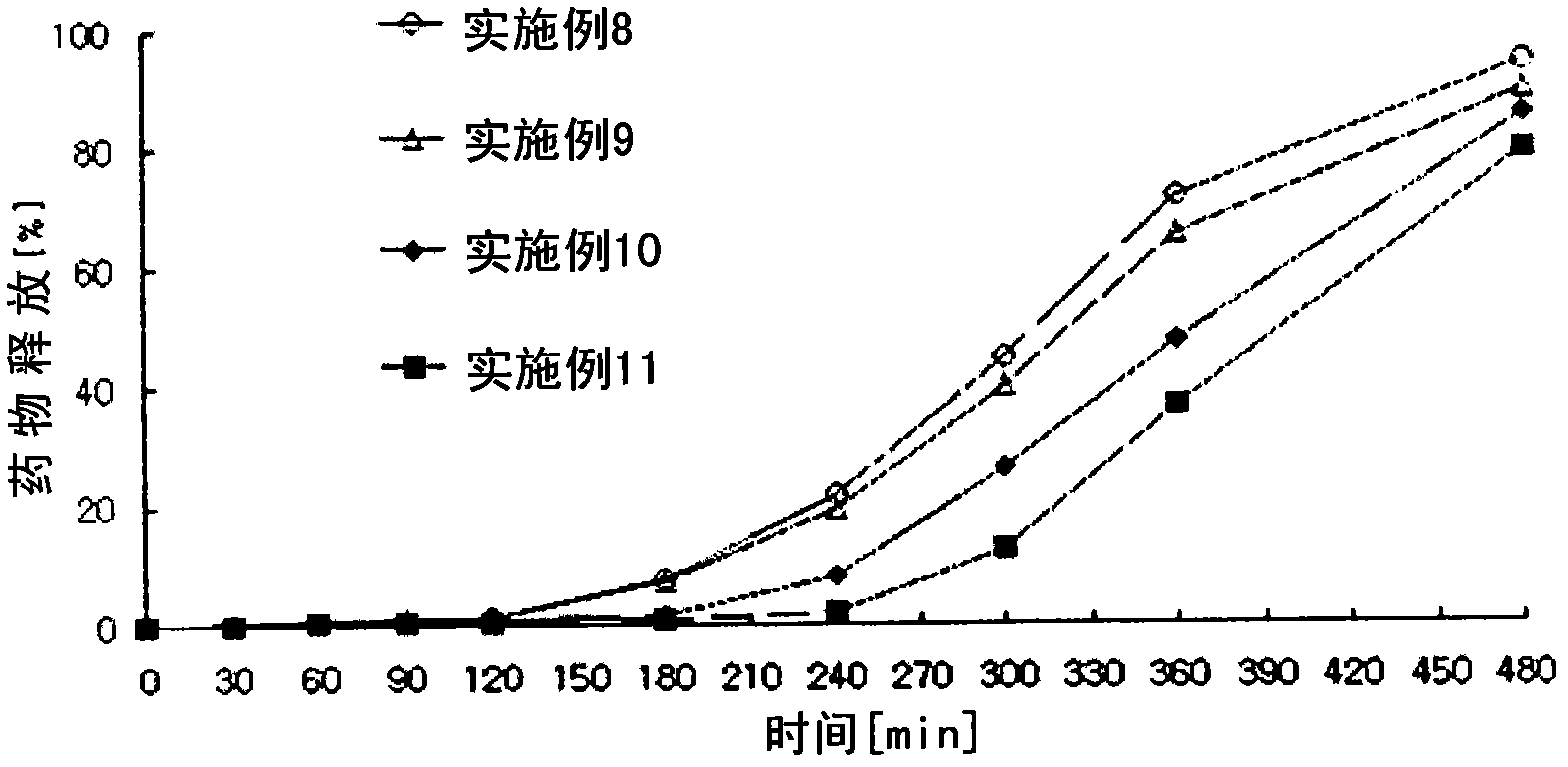
Examples
Embodiment 1
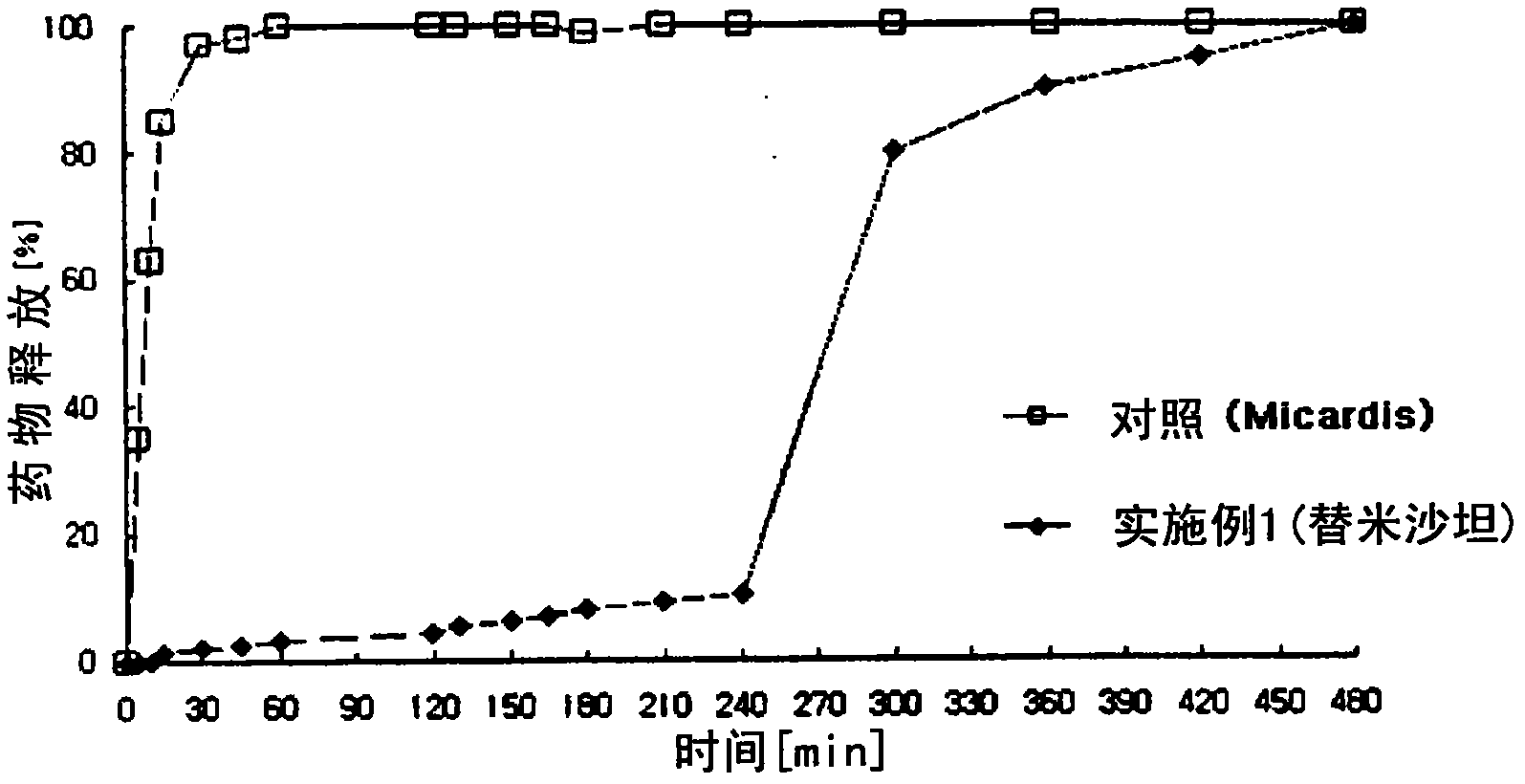
[0148] Preparation of uncoated tablet containing angiotensin-II-receptor blocker
[0149] 1) Preparation of angiotensin-II-receptor blocker sustained-release granules
[0150] According to the content of ingredients shown in Table 1 below, Telmisartan, microcrystalline cellulose, sorbitol, meglumine and sodium hydroxide were sieved through a No. 35 sieve and mixed in a high-speed mixer for 5 minutes to prepare mixture. Meanwhile, polyvinylpyrrolidone was dissolved in purified water (70 mg / tablet) to prepare a binding solution, followed by kneading, granulation and drying. The dried material was placed in a fluid bed coater. Meanwhile, polyvinyl acetate was dissolved in a mixed solvent of ethanol and dichloromethane (2:8) (240 mg / tablet) to prepare a solution, which was then coated in a fluidized bed coater (GPCG-1: Glatt, Germany ) coated on the particles. After the coating process is completed, drying is performed to prepare angiotensin-II-receptor blocker sustained-...
Embodiment 2
[0153] Preparation of uncoated tablet containing angiotensin-II-receptor blocker
[0154] 1) Preparation of angiotensin-II-receptor blocker sustained-release granules
[0155] According to the ingredient content shown in Table 1 below, irbesartan, pregelatinized starch, lactose and croscarmellose sodium were sieved through a No. 35 sieve and mixed in a high-speed mixer for 5 minutes to prepare mixture. Meanwhile, Poloxamer 188 was dissolved in purified water (90 mg / tablet) to prepare a binding solution, followed by kneading, granulation and drying. The dried material was placed in a fluid bed coater. Meanwhile, cellulose acetate (acetyl 32.0%) and cellulose acetate (acetyl 39.8%) were dissolved in a mixed solvent (2:8) of ethanol and dichloromethane (800 mg / tablet) to prepare a solution, which was then The granules were coated in a fluid bed coater (GPCG-1: Glatt, Germany). After finishing the coating process, it is dried. The dried material, microcrystalline cellulo...
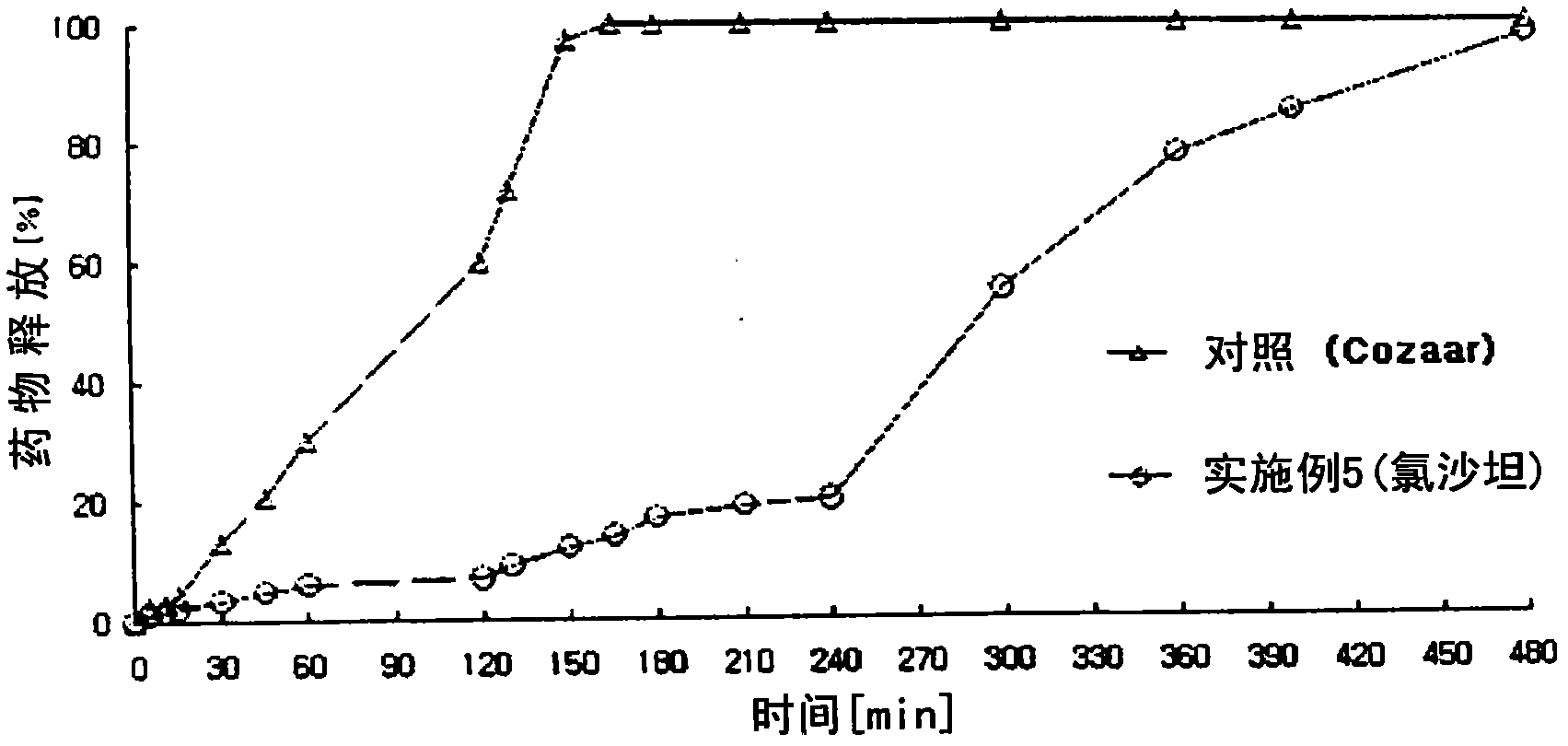
Embodiment 3
[0158] Preparation of uncoated tablet containing angiotensin-II-receptor blocker
[0159] 1) Preparation of angiotensin-II-receptor blocker sustained-release granules
[0160]According to the ingredient contents shown in Table 1 below, valsartan, microcrystalline cellulose, and crosslinked polyvinylpyrrolidone were sieved through a No. 35 sieve, and mixed in a high-speed mixer for 5 minutes to prepare a mixture. Meanwhile, polyvinylpyrrolidone was dissolved in purified water (100 mg / tablet) to prepare a binding solution, followed by kneading, granulation and drying. The dried material was placed in a fluid bed coater. Simultaneously, prepare respectively the solution of hydroxypropyl methylcellulose in purified water (70mg / sheet) and the mixed solvent (8: 2) of hydroxypropylmethylcellulose phthalate in ethanol and purified water ( 120mg / tablet). The granules were placed in a fluidized bed coater (GPCG-1: Glatt, Germany), and then coated in two steps with the prepared s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com