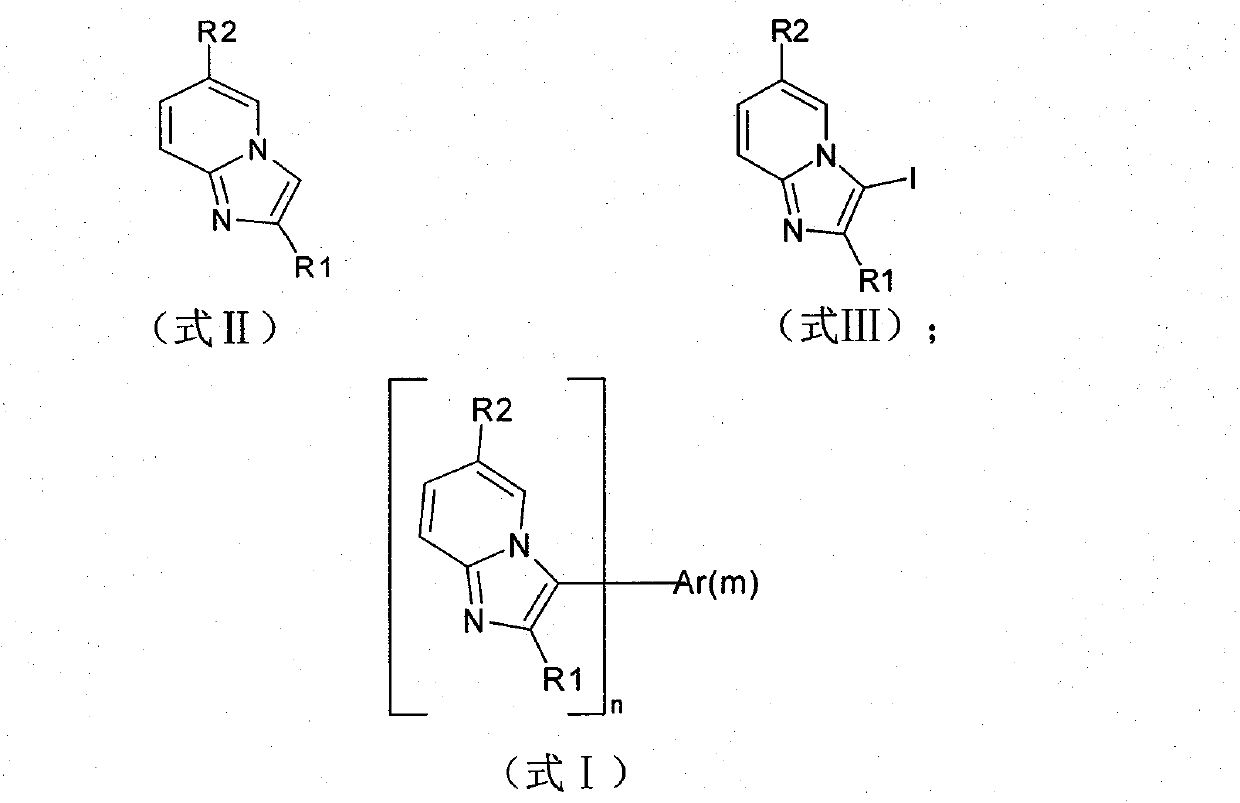
Pyridoimidazole derivatives and application thereof in organic light-emitting devices (OLEDs)
A technology of organic boronic acid and reaction, which is applied in the field of organic electroluminescence materials, and can solve the problem that the position of the emission peak is biased towards ultraviolet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、1
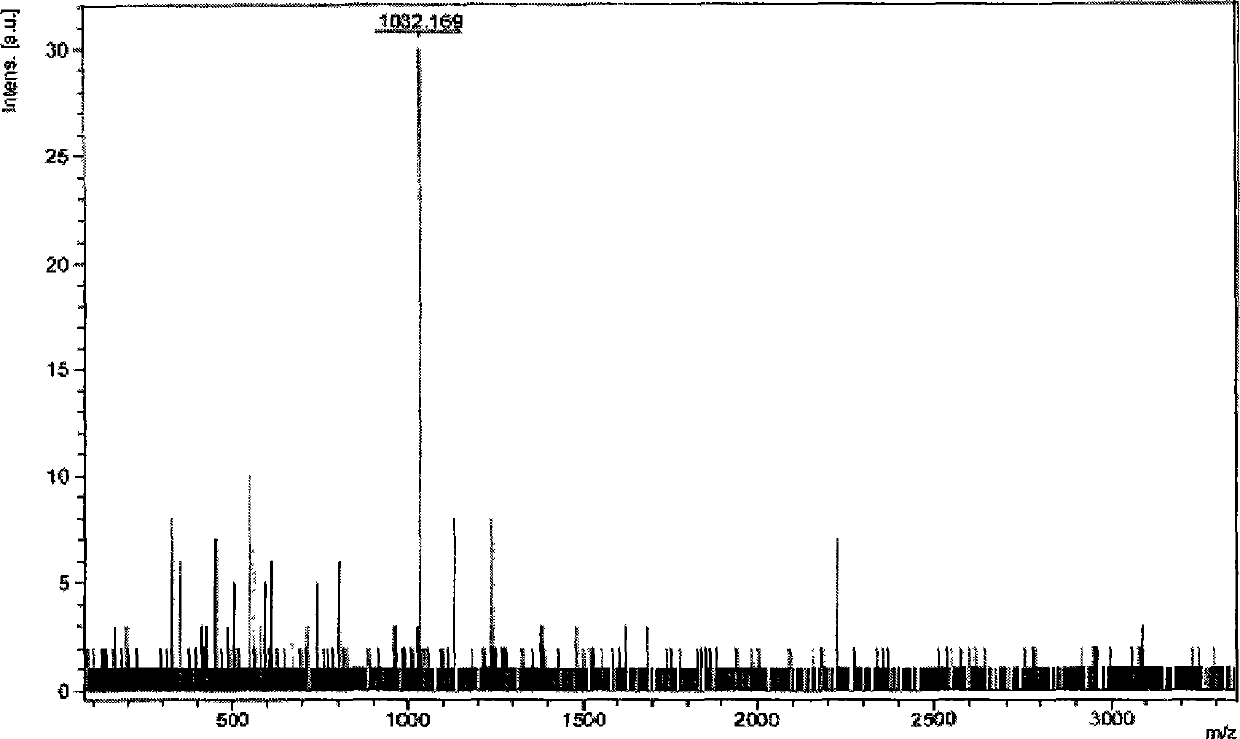
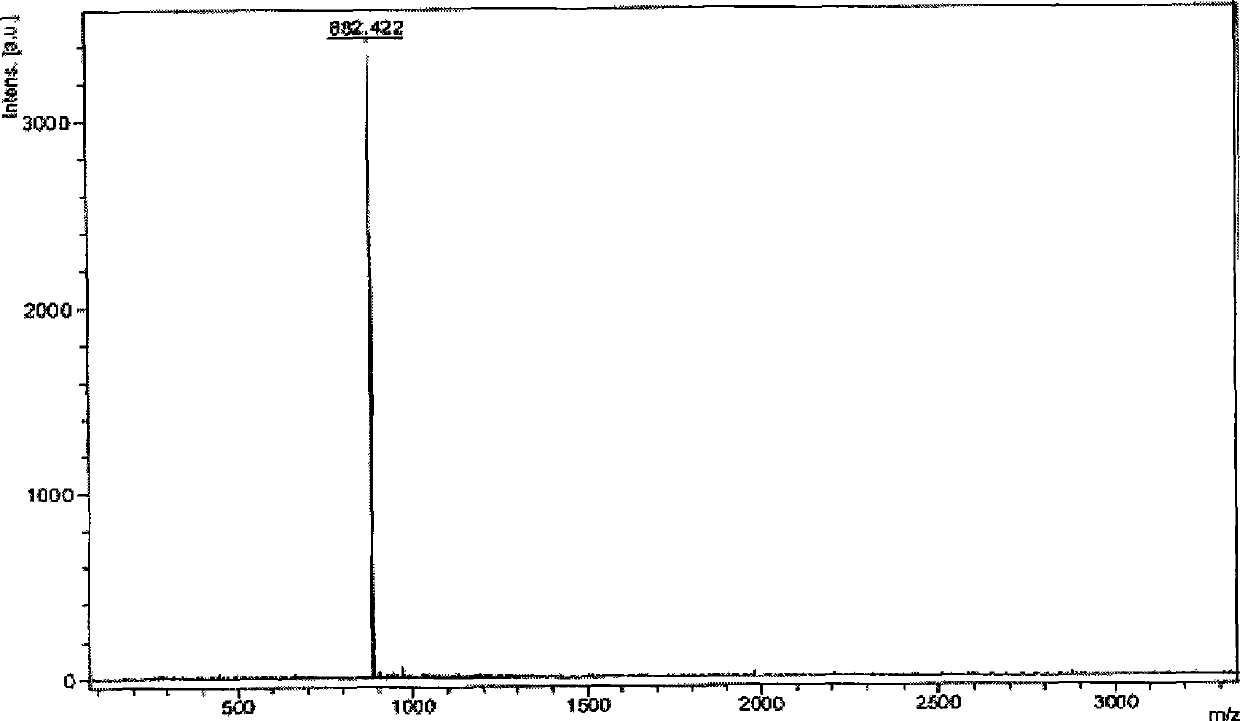
[0075] Embodiment 1,1,3, the preparation of 5-three-(3-(2-phenylpyridoimidazolyl)) benzene
[0076]
[0077] The first step: take 2-phenylpyridimidazole and elemental iodine with a molar ratio of 1:1.5 as raw materials, dissolve the above-mentioned raw materials in pyridine, stir at 50° C. for 6 hours, add a large amount of water, and dichloromethane After extracting and merging the organic phases, the organic phases were washed with water, saturated sodium thiosulfate solution and brine successively, and the organic phases were spin-dried and separated and purified by silica gel column chromatography (eluent was dichloromethane) to obtain the corresponding iodine Substitutes, the yield is about 85%.
[0078] The second step: under the protection of nitrogen, put the iodide obtained in the first step and 1,3,5-triphenylboronic acid (the molar ratio is 5:1) into a two-necked bottle, and add a catalytic amount of tetrakis(triphenyl) Phosphine palladium) and mixed solvent (so...
Embodiment 2
[0091] Embodiment 2, the preparation of 4-[(3-(2-phenylpyridoimidazolyl))-1,3,5-triphenyl]benzene
[0092]
[0093] The first step: take 2-phenylpyridimidazole and elemental iodine with a molar ratio of 1:1.5 as raw materials, dissolve the above-mentioned raw materials in pyridine, stir at 50° C. for 6 hours, add a large amount of water, and dichloromethane After extracting and merging the organic phases, the organic phases were washed with water, saturated sodium thiosulfate solution and brine successively, and the organic phases were spin-dried and separated and purified by silica gel column chromatography (eluent was dichloromethane) to obtain the corresponding iodine Substitutes, the yield is about 85%.
[0094]The second step: under the protection of nitrogen, put the iodide obtained in the first step and 4-(1,3,5-triphenyl) phenylboronic acid (the molar ratio is 5:1) into a two-necked bottle, and add a catalytic amount of Tetrakis (triphenyl) phosphine palladium) and...
Embodiment 3
[0105] Preparation of Example 3.4-[(3-(2-naphthylpyridoimidazolyl))-1,3,5-triphenyl]benzene
[0106]
[0107] The first step: take 2-naphthylpyridoimidazole and elemental iodine with a molar ratio of 1:1.5 as raw materials, dissolve the above raw materials in pyridine, stir at 50°C for 6 hours, add a large amount of water, and dichloromethane After extracting and merging the organic phases, the organic phases were washed with water, saturated sodium thiosulfate solution and brine successively, and the organic phases were spin-dried and separated and purified by silica gel column chromatography (eluent was dichloromethane) to obtain the corresponding iodine Substitutes, the yield is about 85%.
[0108] The second step: under the protection of nitrogen, put the product obtained in the first step and 4-(1,3,5-triphenyl)phenylboronic acid (the molar ratio is 5:1) into a two-necked bottle, and add a catalytic amount of four (triphenyl) phosphine palladium) and mixed solvent (so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com