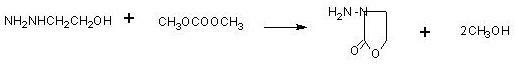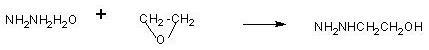Synthesis method of furazolidone
A synthesis method and furazolidone technology, applied in the field of organic chemical synthesis, can solve the problems of complex equipment and low yield, and achieve the effects of simple equipment, high product yield and equipment saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] First add 250g of 80% hydrazine hydrate, raise the temperature to 70°C, and start to vaporize ethylene oxide. The ethylene oxide gas passes through a 5-meter coil, and the coil is placed in 70°C water, and the hot ethylene oxide gas is passed into In hydrazine hydrate, control the aeration speed, the temperature of hydrazine hydrate is 70°C, the time of aeration of 23.7g is about 1.5 hours, and the temperature is kept at 70°C for 1 hour. Then, 60g of hydrazine hydrate was distilled off at atmospheric pressure (the content is more than 50%, and the hydrazine hydrate content was more than 70% after steaming part of the water), and then the hydrazine hydrate was collected by vacuum distillation (the content was above 70°C, the hydrazine hydrate was always Apply mechanically), until the temperature reaches 135°C, evaporate the hydrazine hydrate to dryness as much as possible. 40 g of β-hydroxyethylhydrazine was obtained.
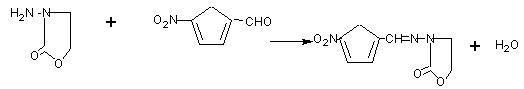
[0039]Cool 40g of β-hydroxyethylhydrazine to 50°C,...
Embodiment 2
[0043] First add 250g of 80% hydrazine hydrate, raise the temperature to 65°C, and start to vaporize ethylene oxide. The ethylene oxide gas passes through a 3-meter coil, and the coil is placed in 50°C water, and the hot ethylene oxide is passed through to hydrate In hydrazine, control the aeration rate, the temperature of hydrazine hydrate is 65°C, the time of aeration of 23.7g is about 1.5 hours, and the temperature is kept at 65°C for 1 hour. Then, 60g of hydrazine hydrate was distilled off at atmospheric pressure (the content is more than 50%, and the hydrazine hydrate content was more than 70% after steaming part of the water), and then the hydrazine hydrate was collected by vacuum distillation (the content was above 70°C, the hydrazine hydrate was always Applicable), until the temperature reaches 140°C, evaporate the hydrazine hydrate to dryness as much as possible. 40 g of β-hydroxyethylhydrazine was obtained.
[0044] Cool 40g of β-hydroxyethylhydrazine to 50°C, first...
Embodiment 3
[0048] First add 285.7g of 70% recovered hydrazine hydrate, and start to vaporize ethylene oxide. The ethylene oxide gas passes through a 100-meter coiled pipe, and the coiled pipe is placed in 70°C water, and the hot ethylene oxide is passed into the hydrazine hydrate. Control the aeration speed, the temperature of hydrazine hydrate is at 70°C, the aeration time of 23.7g is about 1.5 hours, and the temperature is kept at 70°C for 1 hour. Then, 60g of hydrazine hydrate was distilled off at atmospheric pressure (the content is more than 50%, and the hydrazine hydrate content was more than 70% after steaming part of the water), and then the hydrazine hydrate was collected by vacuum distillation (the content was above 70°C, the hydrazine hydrate was always Applicable), until the temperature reaches 130°C, evaporate the hydrazine hydrate to dryness as much as possible. 40 g of β-hydroxyethylhydrazine was obtained.
[0049] Cool 40g of β-hydroxyethylhydrazine to 50°C, first add 8....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com