Allopurinol dual-release preparation and preparation method thereof
An allopurinol and double-release technology, which is applied in pill delivery, pharmaceutical formulations, bone diseases, etc., can solve the problem of large fluctuations in the peak blood drug concentration of ordinary tablets, the inability to maintain a stable and effective anti-gout blood drug concentration, and prone to adverse reactions And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
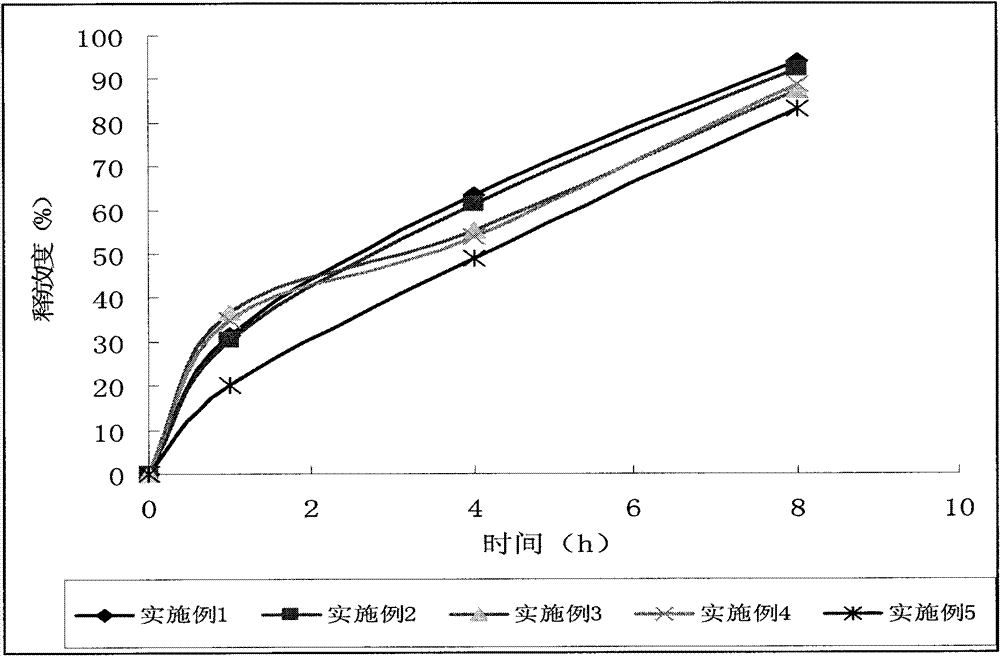
Examples
Embodiment 1
[0026] quick release part
[0027] Allopurinol 50g
[0028] Microcrystalline Cellulose 101 20g
[0029] Starch 30g
[0030] Povidone K30 3g
[0031] Magnesium stearate 0.5g
[0032] Sustained release part
[0033] Allopurinol 200g
[0034] Hypromellose K100LV 40g
[0035] Microcrystalline Cellulose 101 50g
[0036] Povidone K30 15g
[0037] Magnesium stearate 0.8g
[0038] Made into 1000 pieces / grain
[0039] Preparation:
[0040] (1) Preparation of immediate-release granules: crush allopurinol through an 80-mesh sieve, and set aside; weigh allopurinol, starch, povidone K30, and microcrystalline cellulose 101 of the prescribed amount, mix well, add appropriate amount of water, and carry out Wet granulation, wet granulation with a 16-mesh sieve, drying at 50°C, granulation with a 20-mesh sieve, calculate the yield, add magnesium stearate, mix, and set aside.
[0041] (2) Preparation of sustained-release granules: crush allopurinol through an 80-mesh sieve, and set a...
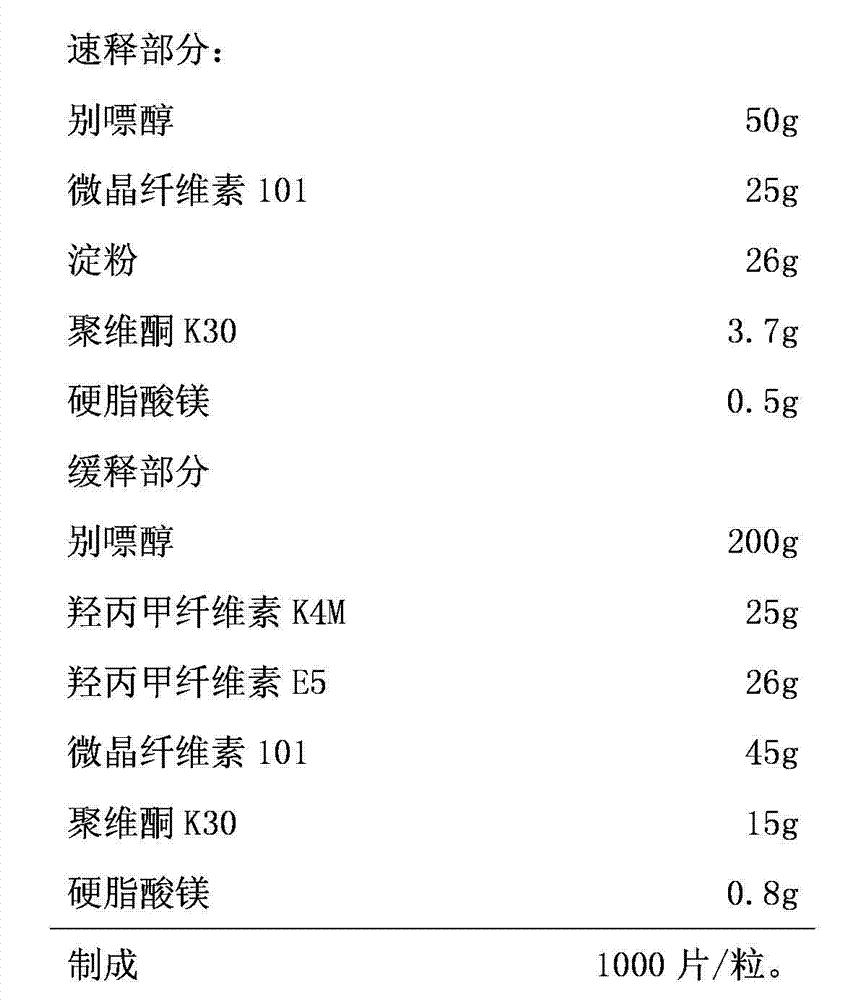
Embodiment 2
[0045] Quick release part:
[0046] Allopurinol 50g
[0047] Microcrystalline Cellulose 101 25g
[0048] Starch 26g
[0049] Povidone K30 3.7g
[0050] Magnesium stearate 0.5g
[0051] Sustained release part
[0052] Allopurinol 200g
[0053]Hypromellose K4M 25g
[0054] Hypromellose E5 26g
[0055] Microcrystalline Cellulose 101 45g
[0056] Povidone K30 15g
[0057] Magnesium stearate 0.8g
[0058] Made into 1000 pieces / grain
[0059] Preparation method: with embodiment 1.
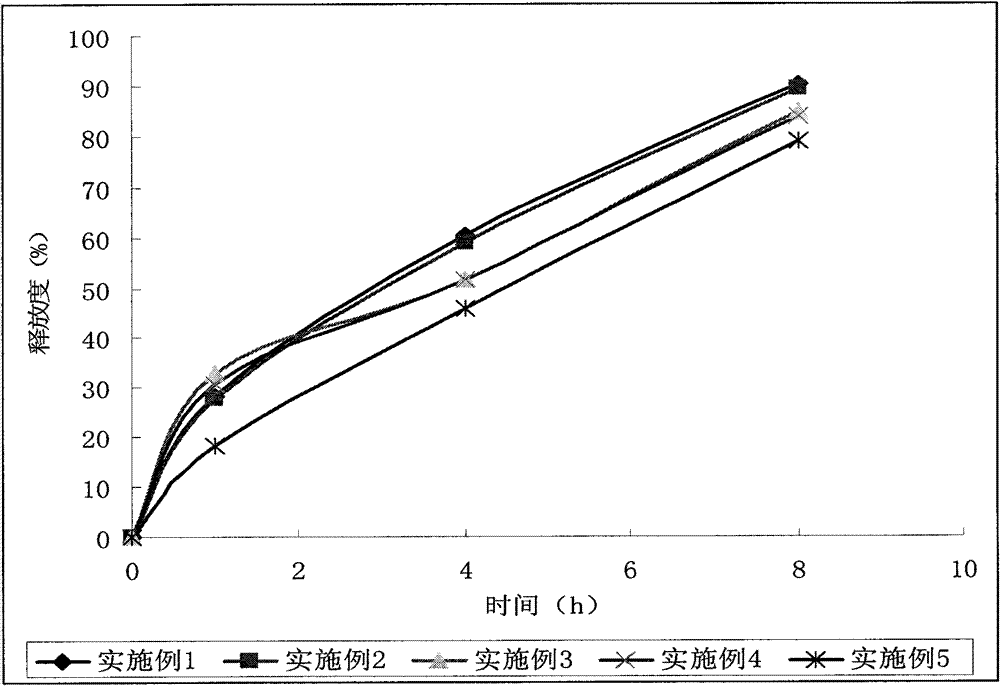
Embodiment 3
[0061] Quick release part:
[0062] Allopurinol 125g
[0063] Microcrystalline Cellulose 101 38g
[0064] Sodium carboxymethyl starch 23g
[0065] Povidone K 30 12g
[0066] Magnesium Stearate 1g
[0067] Sustained release part:
[0068] Allopurinol 125g
[0069] Hypromellose K100LV 55g
[0070] Microcrystalline Cellulose 101 40g
[0071] Povidone K 30 15g
[0072] Magnesium Stearate 1g
[0073] A total of 1000 pieces / grain
[0074] Preparation method: with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com