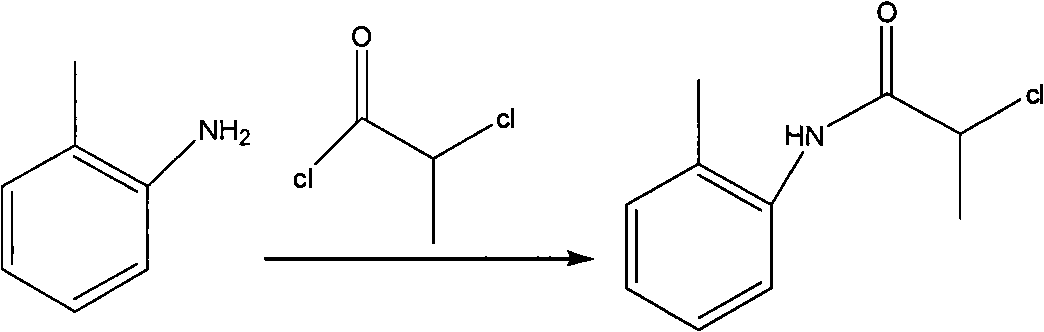
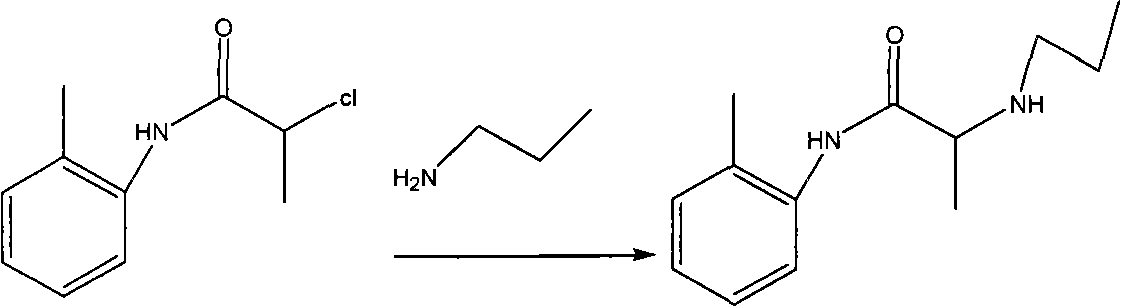
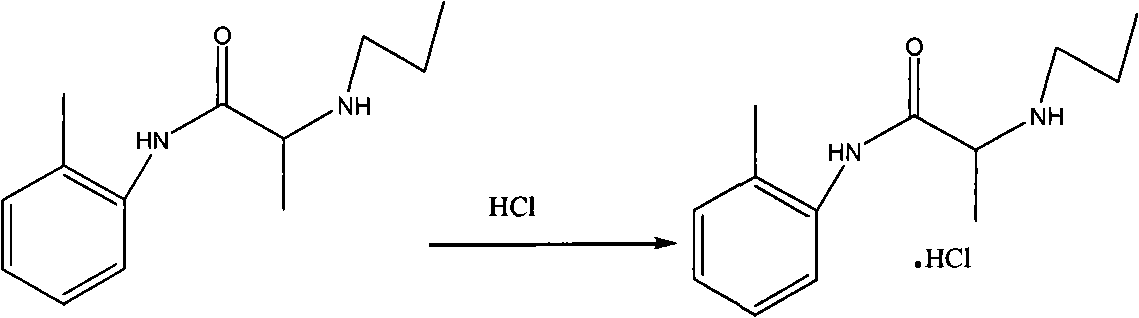
Method for preparing N-(2-Methylphenyl)-2-(propylamino)propa-namide
A technology of prilocaine hydrochloride and prilocaine, which is applied in the field of drug synthesis, can solve the problems of reduced yield, suboptimal synthesis process, unfavorable large-scale industrial production, etc., and achieves high yield, simple operation, and easy raw materials The effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The first step: amidation of o-toluidine
[0028] Add 30kg of o-methylaniline and 58kg of potassium carbonate into the reactor, add 150L of acetone to the reactor in an ice-water bath, slowly add 53kg of 2-chloropropionyl chloride dropwise, and control the internal temperature at 20-30°C for two hours The dropwise addition was completed; a large amount of solids were precipitated during the dropwise addition; reaction at room temperature. After detecting the disappearance of the raw material point (TLC detection, developing system: V dichloromethane: V petroleum ether=1: 3, add 2 drops of glacial acetic acid), stop the reaction, the reaction time is 2h; add 200L water in the reactor, and A large amount of solids were precipitated, and mechanical stirring was used to break the solids as much as possible, filtered, washed with 10 L of water each time, a total of 4 times, and dried, and the obtained solids were placed in a blast oven at 60 ° C for 11 hours to obtain 51 kg ...
Embodiment 2
[0034] The first step: amidation of o-toluidine
[0035] Add 30kg of o-methylaniline and 39kg of potassium carbonate into the reactor, add 150L of acetone to the reactor in an ice-water bath, slowly add 35kg of 2-chloropropionyl chloride dropwise, and control the internal temperature at 20-30°C for two hours The dropwise addition was completed; a large amount of solids were precipitated during the dropwise addition; reaction at room temperature. After detecting the disappearance of raw material points (TLC detection, developing system: V dichloromethane: V petroleum ether = 1: 3, add 2 drops of glacial acetic acid), stop the reaction, the reaction time is 3h; add 200L water in the reactor, and A large amount of solids were precipitated, and mechanical stirring was used to break the solids as much as possible, filtered, washed with 10 L of water each time, a total of 4 times, and dried, and the obtained solids were placed in a blast oven at 60 ° C for 11 hours to obtain 42 kg o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com