Synthesis method of spiro orthoester monomer
A technology of spiro-orthoester and synthesis method, which is applied in the direction of organic chemistry, can solve problems such as attack and environmental damage, and achieve the effects of reducing volume shrinkage, preventing material aging, and improving bonding effect
Inactive Publication Date: 2011-06-15
SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
View PDF1 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Residual stress will also make cemented joints or resin and fiber interfaces susceptible to attack by environmental factors such as oxygen and water, resulting in premature environmental damage
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
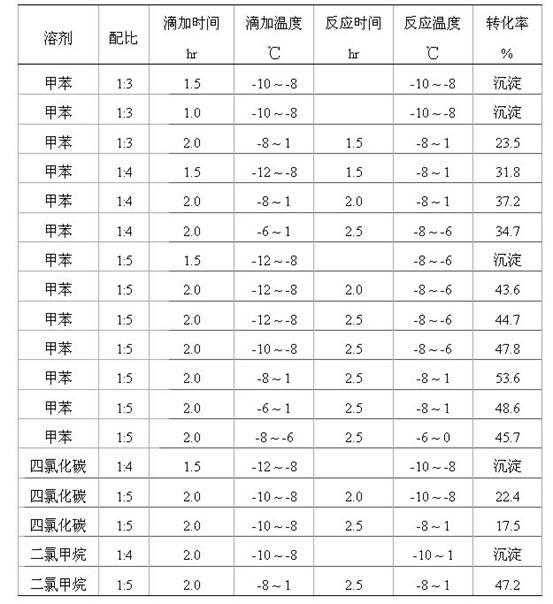
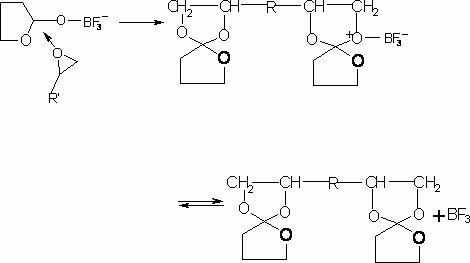
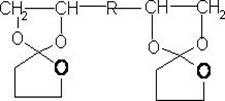
The invention provides a synthesis method of a spiro orthoester monomer, relating to a polymer synthesis method. The synthesis method comprises the following steps: adding toluene and gamma-butyrolactone in a three-mouth flask; fully dissolving E-54 with toluene, and then dissolving 20.66mL of catalyst BF3.OEt in toluene; after the temperature of the device is cooled to -12 DEG C by cryohydrate bath and a first batch of catalyst is added, dropwise adding a resin toluene solution; adding a terminator triethylamine to terminate the reaction; carrying out post-processing: preparing a NaOH solution, and washing with the NaOH solution; adding magnesium sulfate in the reaction liquid for drying, and then filtering; carrying out distillation at reduced pressure so as to obtain a milkiness thick liquid; and performing vacuum drying so as to obtain the milk-white wax-shaped spiro orthoester monomer. The spiro orthoester monomer is copolymerized with a resin adhesive, which has important significance in the aspects of improving the adhesion effect, preventing the material from aging, reducing the volume contractibility rate during curing, improving the strength of thermosetting resins, prolonging the service life and the like.
Description
A kind of synthetic method of spiro ring ortho ester monomer technical field The invention relates to a method for synthesizing a polymer, in particular to a method for synthesizing a spirocyclic ortho ester monomer. Background technique In August 1974, Bailey, an American scientist, read the paper "Polymerization of Spirocyclic Ether Monomers into Polycarbonate Containing Ether Bonds" at the Japan-US Science Symposium, and first proposed the expansion polymerization reaction. Swelling polymerization refers to a type of polymerization that can produce volume expansion during the polymerization process. It is an important branch of ring-opening polymerization, which is the result of the increase in molecular size caused by cationic or free radical ring-opening polymerization of spirocyclic and bicyclic orthoesters. The monomers capable of this polymerization reaction are usually called expansion monomers. The earliest known expanding monomer was 1,4,6-trioxaspiro[4,4]nona...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D493/10C07D519/00
Inventor 王长松宁志高梁兵李安东刘大晨
Owner SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com