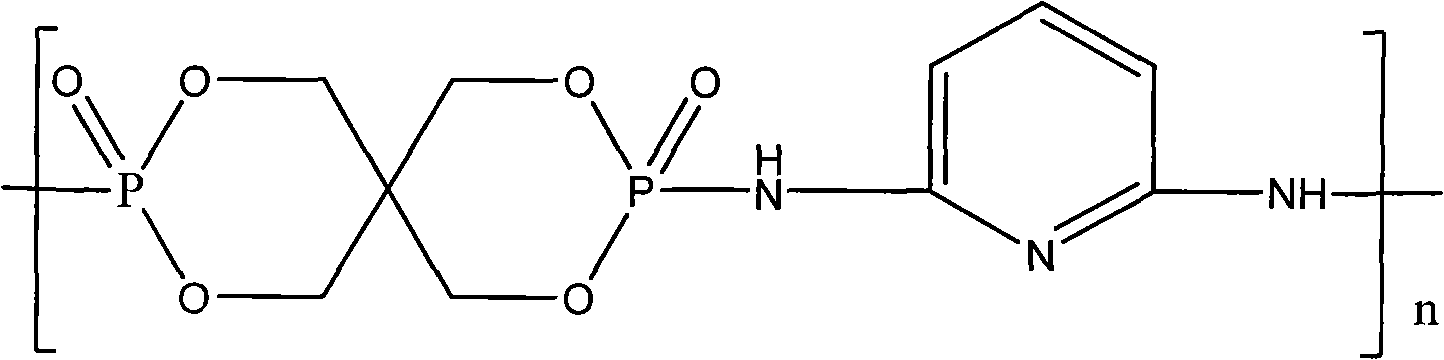
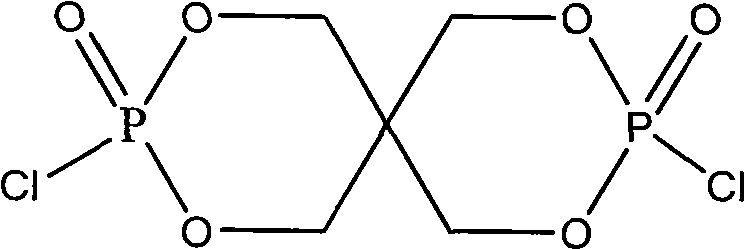
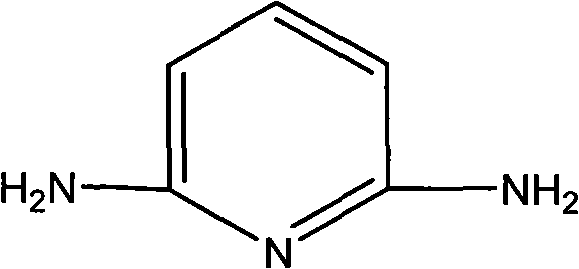
Macromolecular intumescent flame retardant with phosphorus and nitrogen and synthesis method thereof
An intumescent flame retardant and a synthesis method technology, applied in the field of intumescent halogen-free flame retardants, can solve the problems of large molecular weight, etc., and achieve the effects of no halogen, good compatibility, and high carbon-forming properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Add 59.4g of chlorinated spirocyclic phosphate and 100mL of acetonitrile into a 250mL reactor equipped with a nitrogen protection and stirring device, heat to 50°C to 60°C and keep stirring to completely dissolve the chlorinated spirocyclic phosphate in Acetonitrile solvent;
[0023] (2) Get 19g of 2,6-diaminopyridine and dissolve it in 60mL of acetonitrile solvent to make a concentration of 2.9mol / L containing 2,6-diaminopyridine in acetonitrile solution, which contains 2,6-diaminopyridine The acetonitrile solution is added dropwise in the solution of step (1) gained;
[0024] (3) Add 15 mL of catalyst pyridine dropwise into the solution obtained in step (2), raise the temperature to 80° C. and react for 4 hours, during which light yellow powder is continuously formed;
[0025] (4) Cool to room temperature, filter the reaction product, and wash the filter cake repeatedly with ethanol for 3 to 5 times to purify the product;
[0026] (5) Vacuum drying at 80°C for 1...
Embodiment 2
[0028] (1) Add 59.4g of chlorinated spirocyclic phosphate and 120mL of acetonitrile into a 250mL reactor equipped with a nitrogen protection and stirring device, heat to 50°C to 60°C and keep stirring to completely dissolve the chlorinated spirocyclic phosphate in Acetonitrile solvent;
[0029] (2) Get 21g of 2,6-diaminopyridine and dissolve it in 40mL of acetonitrile solvent to make a concentration of 4.9mol / L containing 2,6-diaminopyridine in acetonitrile solution, which contains 2,6-diaminopyridine The acetonitrile solution is added dropwise in the solution of step (1) gained;
[0030] (3) Add 20 mL of catalyst triethylamine dropwise into the solution obtained in step (2), raise the temperature to 80° C. and react for 6 hours, during which light yellow powder is continuously formed;
[0031] (4) Cool to room temperature, filter the reaction product, and wash the filter cake repeatedly with water for 3 to 5 times to purify the product;
[0032] (5) Vacuum drying at 80°C fo...
Embodiment 3
[0034] (1) Add 29.7g of chlorinated spirocyclic phosphate and 80mL of acetonitrile into a 250mL reactor equipped with a nitrogen protection and stirring device, heat to 50°C to 60°C and keep stirring to completely dissolve the chlorinated spirocyclic phosphate in Acetonitrile solvent;
[0035] (2) Get 9.5g of 2,6-diaminopyridine and dissolve it in 80mL of acetonitrile solvent to make a concentration of 1.1mol / L containing 2,6-diaminopyridine in acetonitrile solution, which contains 2,6-diaminopyridine The acetonitrile solution of pyridine is added dropwise in the solution obtained in step (1);
[0036] (3) Add 10 mL of catalyst pyridine dropwise into the solution obtained in step (2), raise the temperature to 80° C. and react for 3 hours, during which light yellow powder is continuously formed;
[0037] (4) Cool to room temperature, filter the reaction product, and wash the filter cake repeatedly with ethanol for 3 to 5 times to purify the product;
[0038] (5) Vacuum drying...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com