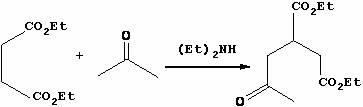Method for preparing trinexapac-ethyl
A technology of trinexapac-ethyl and diethyl succinate, which is applied in the preparation of carboxylic acid esters, the preparation of organic compounds, chemical instruments and methods, etc., and can solve problems such as low product purity, low yield, and harsh reaction conditions , to achieve the effect of high yield, good product purity and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Step 1, the synthesis of 2-acetone-1,4-diethyl succinate
[0026] Put diethyl maleate (1800.0 g, 10.45 mol), acetone (6000 mL) and diethylamine (149.9 g, 2.07 mol) into a 10 L autoclave, heat to 160 °C, keep the reaction for 24 hours, and cool to room temperature , Atmospheric pressure distillation to remove low boilers, and then oil pump maximum vacuum vacuum distillation to collect fractions at 120-140 ℃ to obtain light yellow liquid diethyl 2-acetone-1,4-succinate with a yield of 89.2%;
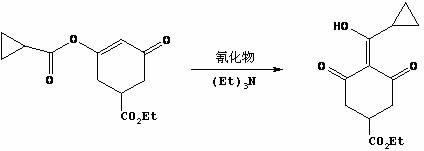
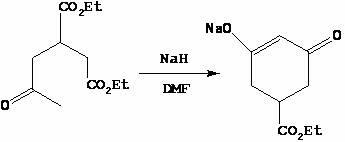
[0027] Step 2, the synthesis of 3-ethoxycarbonyl-5-oxocyclohex-1-en-1-alcohol sodium
[0028] Diethyl 2-acetone-1,4-butanedioate (200.0 g, 0.87 mol) was added dropwise to the reaction solution twice, and the temperature was controlled at 30-60 °C. First, 40.0 g was added dropwise. 90 ℃, add the remaining 160.0 g dropwise, after the dropwise addition, the reaction solution was stirred at 80~90 ℃ for 30~45 minutes, TLC detection, the raw material 2-acetone-1,4-diethyl succinate disa...
Embodiment 2
[0036] Step 1, the synthesis of 2-acetone-1,4-diethyl succinate
[0037] Put diethyl maleate (1800.0 g, 10.45 mol), acetone (6200 mL) and diethylamine (149.9 g, 2.07 mol) into a 10 L autoclave, heat to 165 °C, keep the reaction for 26 hours, and cool to room temperature , Atmospheric pressure distillation to remove low boilers, and then oil pump maximum vacuum vacuum distillation to collect 120 ~ 140 ℃ distillate to obtain light yellow liquid 2-acetone-1,4-diethyl succinate with a yield of 90.1%;
[0038] Step 2, the synthesis of 3-ethoxycarbonyl-5-oxocyclohex-1-en-1-alcohol sodium
[0039] Diethyl 2-acetone-1,4-butanedioate (150.0 g, 0.65 mol) was added dropwise to the reaction solution twice, and the temperature was controlled at 30-60 °C. First, 30.0 g was added dropwise. After the dropwise addition, heated to 80-90 °C, stirred for 0.5 hours, and kept at 80-90 °C ℃, add the remaining 120.0 g dropwise, after the dropwise addition is complete, the reaction solution is stirr...
Embodiment 3
[0047] Step 1, the synthesis of 2-acetone-1,4-diethyl succinate
[0048] Put diethyl maleate (1800.0 g, 10.45 mol, 60wt.%), acetone (6315 mL) and diethylamine (149.9 g, 2.07 mol) into a 10 L autoclave, heat to 162 °C, and keep the temperature for 26 hours , cooled to room temperature, atmospheric distillation to remove low boilers, and then oil pump maximum vacuum vacuum distillation to collect 120 ~ 140 ℃ distillate to obtain light yellow liquid 2-acetone-1,4-diethyl succinate, the yield is 91.2%.
[0049] Step 2, the synthesis of 3-ethoxycarbonyl-5-oxocyclohex-1-en-1-alcohol sodium
[0050] Diethyl 2-acetone-1,4-butanedioate (600.0 g, 2.60 mol) was added dropwise to the reaction liquid twice, and the temperature was controlled at 30-60 °C. First, 120.0 g was added dropwise. ℃, add the remaining 480.0 g dropwise, after the dropwise addition is complete, the reaction solution is stirred at 80~90°C for 30~45 minutes, TLC detection, the raw material 2-acetone-1,4-diethyl succ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com