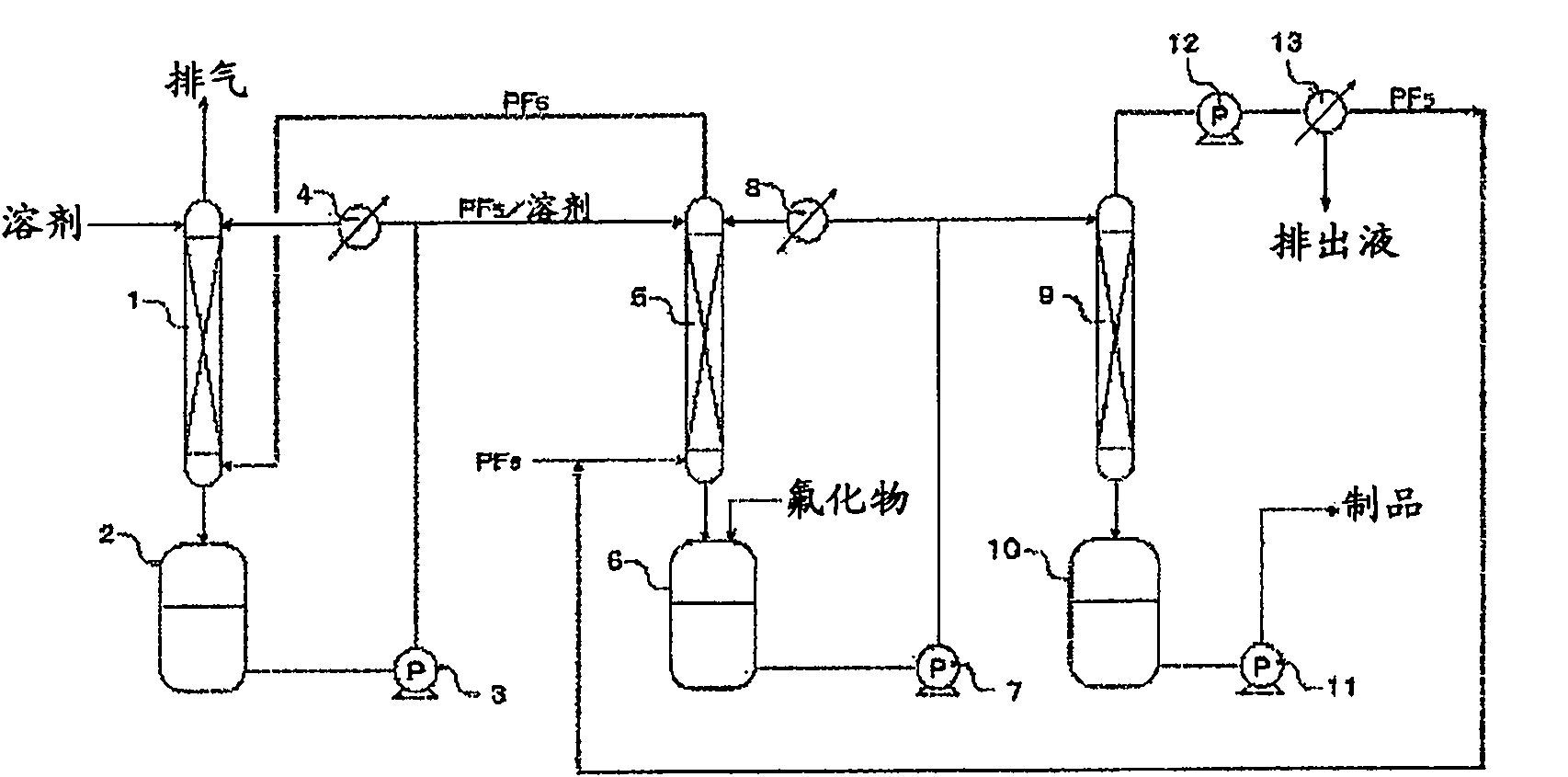
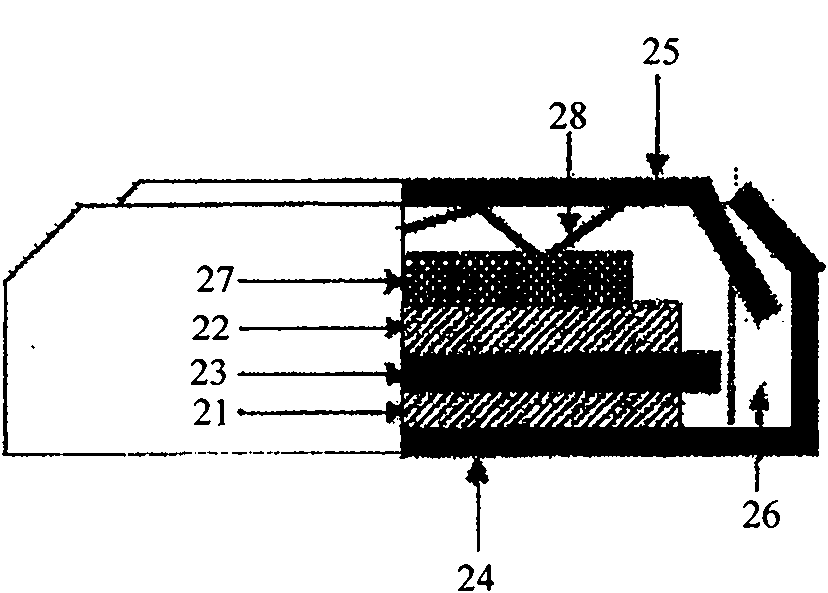
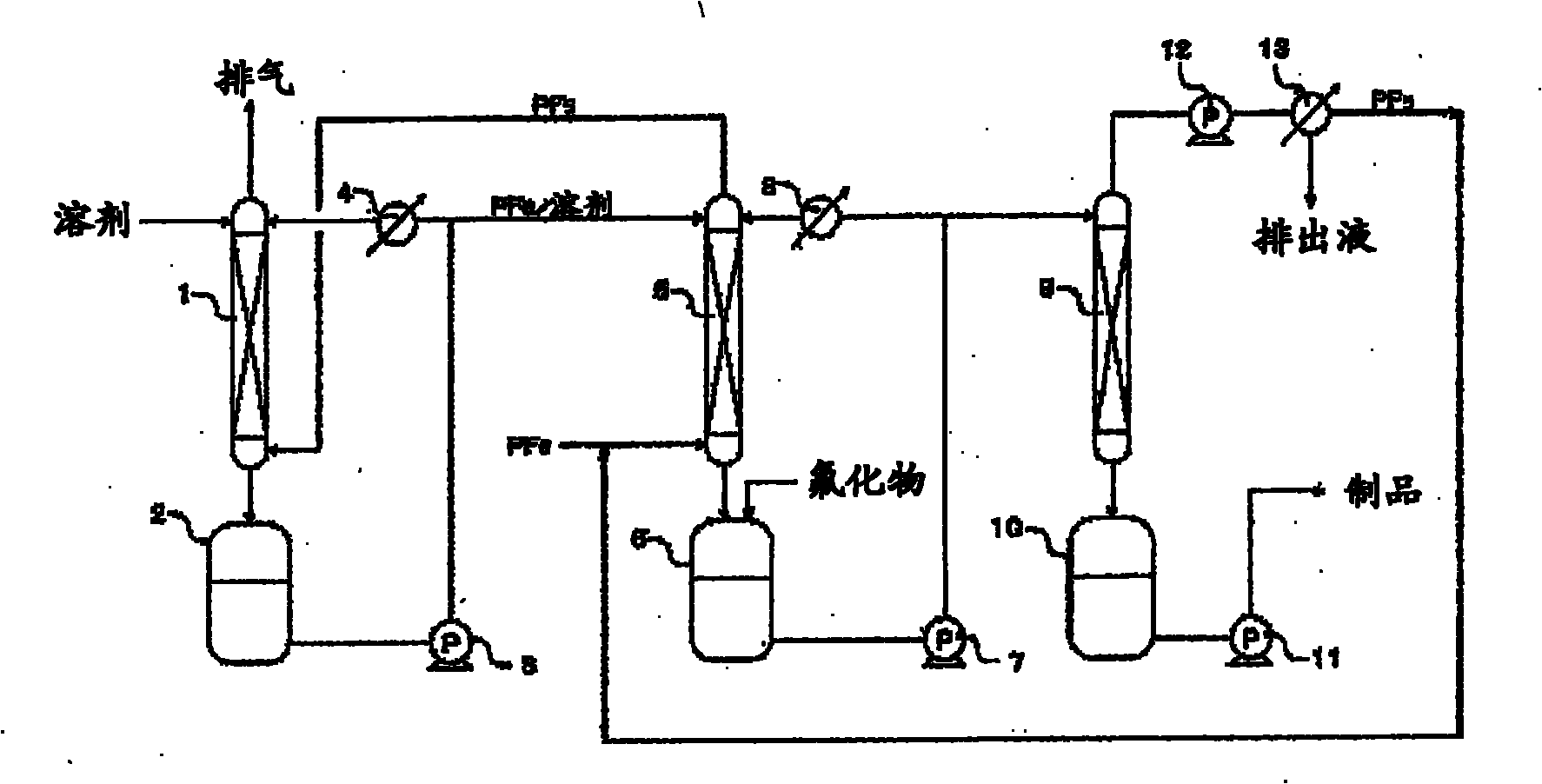
Process for production of hexafluorophosphates
A technology of hexafluorophosphate and manufacturing method, applied in lithium hexafluorophosphate, chemical instruments and methods, phosphorus compounds, etc., can solve the problems of expensive raw materials, high manufacturing cost, poor handling, etc., and achieve high safety and electrical characteristics. great effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] 1000 g of ultrapure water was charged into a 5 L reaction tank made of fluororesin, the reaction tank was heated with an oil bath, and the ultrapure water was kept at 40°C. This ultrapure water was stirred with a rotor, while adding 800 g of commercially available acidic ammonium fluoride (NH 4 F·(HF)), to dissolve it.
[0108] Next, the temperature of the solution in the reaction tank was kept at 40°C, and at the same time, the POF 3 Air bubbles to absorb 740g POF 3 gas. The absorption amount was obtained from the increase in the weight of the reaction solution. absorbing POF 3 After degassing, stirring was continued for 2 hours to bring the temperature of the reaction liquid to 20° C., and then the liquid temperature was kept constant.
[0109] Next, the reaction tank was heated with an oil bath, and the solution in the reaction tank was evaporated to dryness. Residual crystals in the reaction tank were recovered, washed with 750 g of 75% HF aqueous solution, an...
Embodiment 2
[0112] Put 100g of acidic potassium fluoride (KF (HF)) and 500g of semiconductor grade 75% by weight hydrogen fluoride (HF) solution together with the rotor into a 3L reaction tank made of fluororesin (PFA), stir under ice bath to make KF (HF) ) dissolved. Weigh again 140g 85% by weight phosphoric acid (H 3 PO 4 ) solution in a separatory funnel, slowly drop it over 30 minutes under an ice bath, and stir to allow it to react for 6 hours.
[0113] Next, the solution was cooled at -5°C for 24 hours to precipitate crystallization. Thus, a hydrogen fluoride solution in which a precipitate was precipitated was obtained. This hydrogen fluoride solution is filtered off by suction filtration. The liquid temperature of the hydrogen fluoride solution at this time was -5°C. In addition, when the HF concentration of the filtrate was quantified, it was 46% by weight.
[0114] The recovered crystals were washed with 600 g of semiconductor grade 75% by weight hydrogen fluoride (HF) sol...
Embodiment 3
[0119] Put 210g of cesium fluoride (CsF) and 700g of semiconductor-grade 75% by weight hydrogen fluoride (HF) solution together with the rotor into a 3L PFA reaction vessel, and stir in an ice bath to dissolve the CsF. Weigh again 175g 85% by weight phosphoric acid (H 3 PO 4 ) solution in a separatory funnel, slowly drip it in 30 minutes under an ice bath, and stir to make it react for 12 hours.
[0120] Next, the solution was cooled at -5°C for 36 hours to precipitate crystallization. Thus, a solution in which a precipitate had been precipitated was obtained. The solution is then filtered off with suction. The liquid temperature of the phosphoric acid solution at this time was -5 degreeC. In addition, when the HF concentration of the filtrate was quantified, it was 55% by weight.
[0121] The recovered crystals were washed with 400 g of anhydrous hydrofluoric acid cooled to 0°C. Next, the washed filtrate was transferred to a 3L fluororesin flask, and high-purity N was b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| rate of recovery | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com