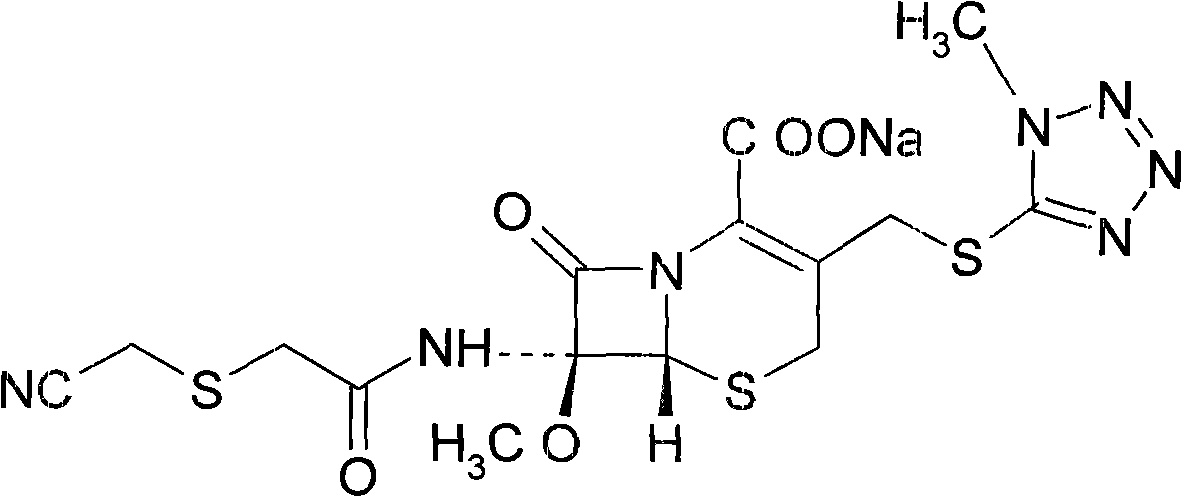
Cefmetazole sodium liposome freeze-dried preparation and preparation method
A technology of cefmetazole sodium lipid and cefmetazole sodium, which is applied in the field of cefmetazole sodium liposome freeze-dried preparation and preparation, can solve the problem of temperature, light, humidity instability, easy precipitation, easy quality change, etc. problems, to achieve good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Cefmetazole sodium 1.0 weight unit
[0026] Sodium chloride 1.5 weight units
[0027] Egg yolk lecithin, distearic acid lecithin, soybean lecithin 1:1 mixed to take 2.5 weight units
[0028] Mannitol 1.0 weight unit
[0029] Sodium hydrogen phosphate 0.5~3.0 weight units
[0030] Weigh the prescription amount of cefmetazole sodium, sodium chloride, and mannitol, mix and dissolve in water, stir evenly, and set aside; Fatty acid lecithin and soybean lecithin are dispersed and dissolved in it, dried under reduced pressure to form a blank film material, added to the above aqueous solution, and ultrasonically treated to the required particle size and uniformity of cefmetazole sodium liposome suspension; container, filling, and vacuum freeze-drying to obtain the cefmetazole sodium liposome freeze-dried preparation.
Embodiment 2
[0032] Cefmetazole sodium 1.0 weight unit
[0033] Sodium chloride 1.5 weight units
[0034] Lecithin disalmitate 5.0 weight units
[0035] Mannitol 1.0 weight unit
[0036] Calcium hydrogen phosphate 0.5~3.0 weight units
[0037] Weigh the prescription amount of cefmetazole sodium, sodium chloride, and mannitol, mix and dissolve in water, stir evenly, and set aside; take an appropriate amount of calcium hydrogen phosphate to prepare 10% buffered saline solution, and dissolve the prescription amount Disperse and dissolve in water, dry under reduced pressure to form a blank membrane material, add to the above aqueous solution, and ultrasonically treat the cefmetazole sodium liposome suspension to the required particle size and uniformity; constant volume, filling, and vacuum freezing After drying, the cefmetazole sodium liposome lyophilized preparation is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com