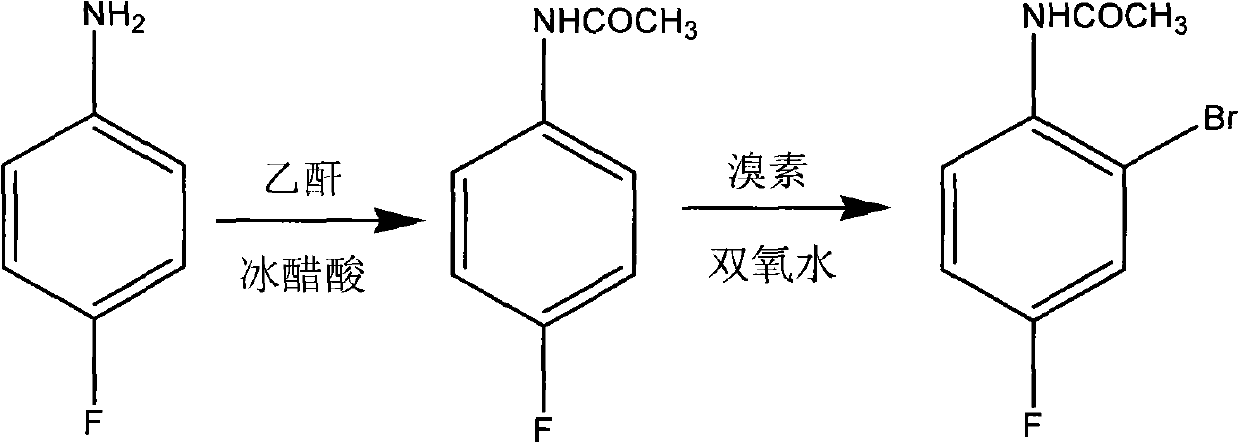
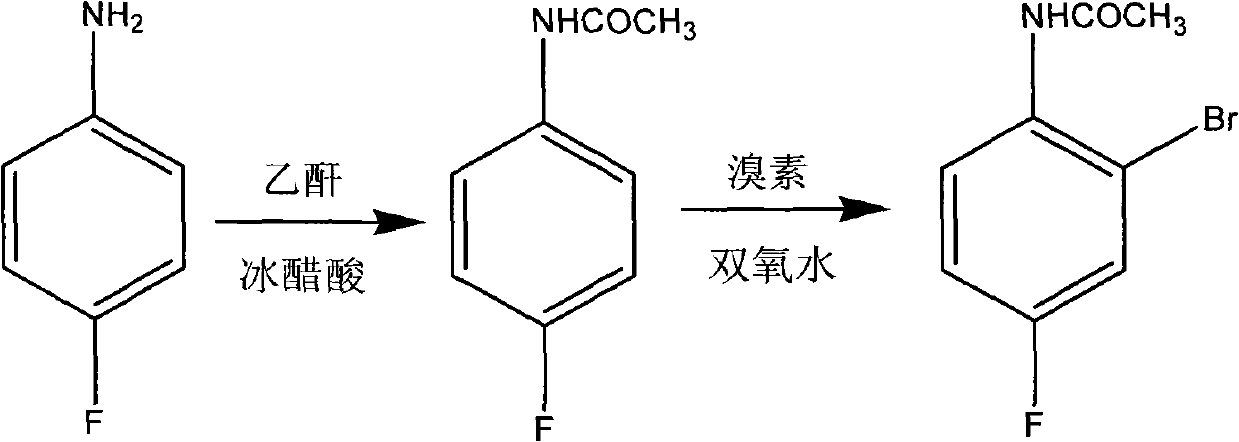
Preparation method of 2-br-4-fluoacetanilide
A technology of fluoroacetanilide and p-fluoroaniline, which is applied in the field of preparation of 2-bromo-4-fluoroacetanilide, can solve the problems of unavailable raw materials and low single-step yield, and achieve reduced production costs, high purity, and easy operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Put 13.9g of p-fluoroaniline into a 250mL four-necked bottle equipped with stirring, thermometer, condenser, and dropping funnel, add 36g of glacial acetic acid under stirring, raise the temperature to 50°C, and add 13g of acetic anhydride dropwise. Then raise the temperature to 55-60°C and keep it for 2h; then cool down to 50-53°C and add 14g of bromine dropwise. Then the temperature was raised to 55-60°C for 1h. After keeping, it was poured into a stirred 250ml aqueous solution containing 2% sodium bisulfite for crystallization, and the crude product was obtained by suction filtration. After refining with 75% ethanol once more, 24 g of finished product was obtained (total yield 82.62%, GC99.95%).
Embodiment 2
[0018] Example 2: Put 69.5 g of p-fluoroaniline into a 1L four-necked bottle equipped with stirring, thermometer, condenser, and dropping funnel, add 113 g of glacial acetic acid while stirring, raise the temperature to 60°C, and add 75 g of acetic anhydride dropwise. Then raise the temperature to 70-80°C and keep for 3h; then cool down to 48-53°C and add 63g of bromine dropwise, after the addition, raise the temperature to 50-60°C and keep for 2h, then cool down to 45-53°C, add 70g of 27% hydrogen peroxide dropwise, Then the temperature was raised to 50-60°C for 3h. After the retention, it was poured into 250 ml of a stirred 4% sodium bisulfite aqueous solution for crystallization, and the crude product was obtained by suction filtration. After refining once with 70% ethanol, 121.5 g of finished product was obtained (total yield 83.64%, GC99.86%).
Embodiment 3
[0019] Example 3: Put 13.9 g of p-fluoroaniline into a 250 mL four-necked bottle equipped with stirring, thermometer, condenser, and dropping funnel, add 23 g of glacial acetic acid while stirring, raise the temperature slightly to 70 ° C, and add 15 g of acetic anhydride dropwise Then raise the temperature to 80-90°C and keep it for 1h; then cool down to 50-55°C and add 13g of bromine dropwise, after the addition, raise the temperature to 55-60°C and keep it for 1h, then cool down to 50-55°C, add 15g of 25% hydrogen peroxide dropwise, Then the temperature was raised to 55-60°C for 1h. After the retention, it was poured into 250 ml of a stirred 6% sodium bisulfite aqueous solution for crystallization, and the crude product was obtained by suction filtration. After refining once with 80% ethanol, 23.8 g of finished product was obtained (total yield 81.93%, GC99.89%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com