Use of ginsenoside Rg1 in preparation of antidepressant
A ginsenoside and anti-depressant technology, applied in the medical and health field, can solve the problem of little anti-depressant effect, achieve the effect of less toxic side effects, high safety, and sufficient sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
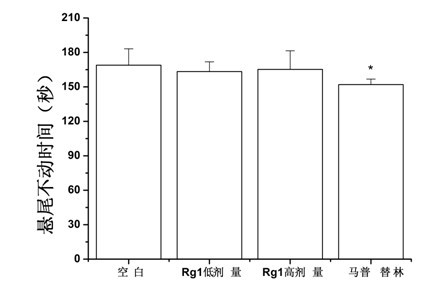
[0038] Example 1 Mouse tail suspension experiment
[0039] 1 Materials and methods
[0040] 1.1 Experimental animals
[0041] SPF-grade Kunming mice, male, weighing 18-22 g, were purchased from the Experimental Animal Center of Sun Yat-sen University School of Medicine, license number: SCXK (Guangdong) 2009-0011.
[0042] Standard feed and litter were purchased from the Experimental Animal Center of Sun Yat-sen University School of Medicine.
[0043] 1.2 Drugs and instruments
[0044] Drugs: Ginsenoside Rg1 was purchased from Yunnan Plant Pharmaceutical Co., Ltd.; the positive drug maprotiline was purchased from Guangzhou Jianmin Pharmacy.
[0045] Automated photographic analysis system for mouse tail suspension experiment; mouse gavage needles for experiments, etc., were purchased from Huaibei Zhenghua Biological Instruments and Equipment Co., Ltd.
[0046] 1.3 Liquid preparation
[0047] Take ginsenoside Rg1 and dissolve it in distilled water, and make solutions with lo...
Embodiment 2
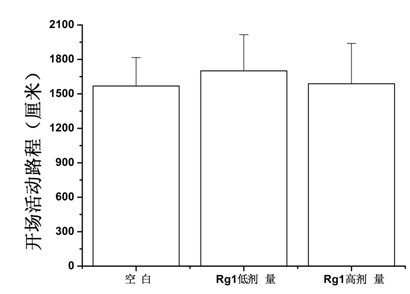
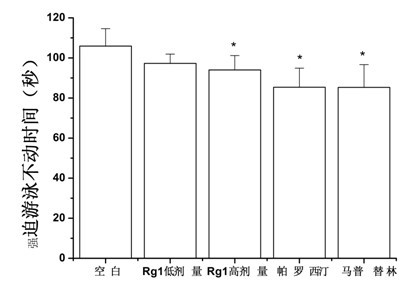
[0056] Example 2 Mice Forced Swimming Experiment
[0057] 1 Materials and methods
[0058] 1.1 Experimental animals
[0059] SPF-grade Kunming mice, male, weighing 18-22 g, were purchased from the Experimental Animal Center of Sun Yat-sen University School of Medicine, license number: SCXK (Guangdong) 2009-0011.
[0060] Standard feed and litter were purchased from the Experimental Animal Center of Sun Yat-sen University School of Medicine.
[0061] 1.2 Instruments and medicines
[0062] Drugs: Ginsenoside Rg1 was purchased from Yunnan Botanical Pharmaceutical Co., Ltd.; positive drugs paroxetine and maprotiline were purchased from Guangzhou Jianmin Pharmacy.
[0063] Instruments: ZH-QPT forced swimming device and analysis system, ZH-QPT opening experiment device and analysis system, mouse gavage needles for experiments, all purchased from Huaibei Zhenghua Biological Instrument Equipment Co., Ltd.; thermometer.
[0064] 1.3 Liquid preparation
[0065] Take ginsenoside Rg1...
Embodiment 3
[0081] Example 3 Acquired Helplessness Experiment
[0082] 1 Materials and methods
[0083] 1.1 Experimental animals
[0084] SPF-grade Kunming mice, male, weighing 18-22 g, were purchased from the Experimental Animal Center of Sun Yat-sen University School of Medicine, license number: SCXK (Guangdong) 2009-0011.
[0085] Standard feed and litter were purchased from the Experimental Animal Center of Sun Yat-sen University School of Medicine.
[0086] 1.2 Instruments and medicines
[0087] Drugs: Ginsenoside Rg1 was purchased from Yunnan Plant Pharmaceutical Co., Ltd.; the positive drug maprotiline was purchased from Guangzhou Jianmin Pharmacy.
[0088] Instruments: BA-200 dark avoidance instrument for mice, purchased from Chengdu Taimeng Technology Co., Ltd.; mouse gavage needles for experiments, purchased from Huaibei Zhenghua Biological Instruments and Equipment Co., Ltd.
[0089] 1.3 Liquid preparation
[0090] Take ginsenoside Rg1 and dissolve it in distilled water, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com