Method for isomerizing alpha-pinene
A technology for isomerization and pinene, which is applied in isomerization to hydrocarbon production, organic chemistry, etc., can solve problems such as environmental pollution and complicated product post-processing, and achieve the effects of high reaction activity, short time consumption and good selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Take 10 ml granular (diameter ≤ 2 mm) high-purity γ-Al 2 o 3 Put it into the glass material fixed bed reaction tube I, in the high-purity N 2 (purity ≥ 99.99%, flow rate 20 -100ml / min) in the atmosphere at 500 o C pretreatment for 2 hours, after that, feed CCl simultaneously at a flow rate of 0.8-2.00 ml / h 4 (pre-dried to remove water), in-situ generation of AlCl 3 ; 5ml of 40-60 mesh mesoporous SiO2 was loaded into the fixed bed reaction tube II 2 , at high purity N 2 300 in airflow (40 ml / min) o After C pretreatment for 2 h, the reaction tube I was connected to the reaction tube II, and the 2 In situ generation of AlCl under gas flow 3 with SiO 2 Reaction of surface hydroxyl groups with AlCl 3 fixed load. After 2-3h, stop feeding CCl 4 , at 300 o Continue to use N under C 2 Purge for 1 h, under N 2 Cool to room temperature under protection for later use. The catalyst is denoted as AlCl 3 / SiO 2 .
Embodiment 2
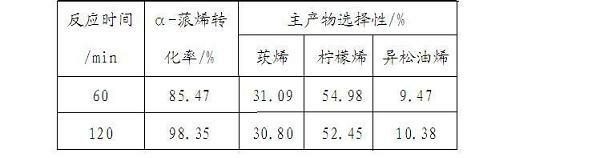
[0021]
[0022] Table 2
Embodiment 3
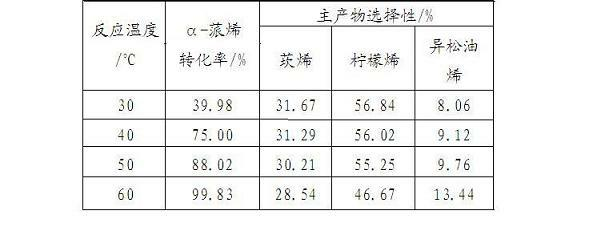
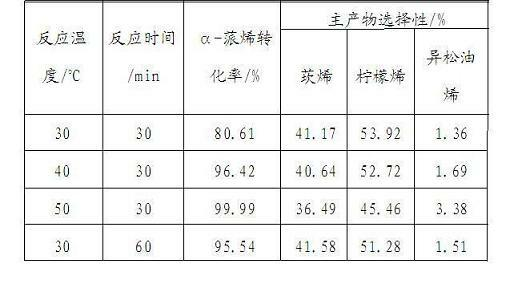
[0024] According to the preparation method of the catalyst in Example 1, the support is changed to double-hole γ-Al 2 o 3 . Carrier in high purity N 2 400 in airflow o After C pretreatment for 2 h, in N 2 Cool down to 300 in airflow o C, with in situ generated AlCl 3 Reaction 3 h, N 2 Warm up to 400 in airflow o C was purged for 1 h, cooled to room temperature for later use, and the obtained catalyst was denoted as AlCl 3 / γ-Al 2 o 3 .
[0025] Use this catalyst to catalyze the isomerization of α-pinene: add magneton, 2 ml α-pinene, 0.2 ml n-decane (internal standard) and 0.1 g AlCl in a dry reaction tube 3 / γ-Al 2 o 3 catalyst. The results at different reaction temperatures and reaction times are shown in Table 3: AlCl 3 / γ-Al 2 o 3 Catalyzed α-pinene isomerization reaction results.
[0026]
[0027] table 3
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com