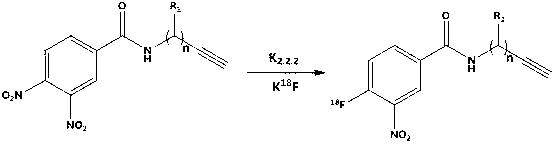
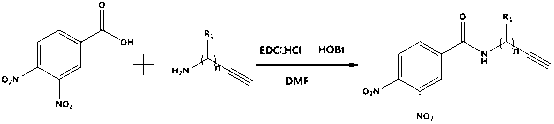
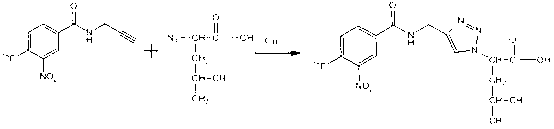
18F labelled precursor compound and preparation method and application thereof
A precursor compound and labeling technology, applied in the preparation of organic compounds, organic chemistry methods, chemical instruments and methods, etc., can solve the problems of unstable metabolism in vivo, long reaction time, low reaction yield, etc., and achieve fast and efficient labeling , the effect of short reaction time and high radioactive yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The synthesis of embodiment 1,3-nitro-4-fluorobenzoyl propargyl amine comprises the following steps successively:
[0044] 1), the synthesis of 3,4-dinitrobenzoyl propargyl amine:
[0045]
[0046] Add 212mg (1mmol) of 3,4-dinitrobenzoic acid, 55mg (1.1mmol) of propynylamine, 288mg (1.5mmol) of EDC·HCl, and 67.5mg (0.5mmol) of HOBt into a 50mL round-bottomed flask in turn, and then add 20mL DMF, react at 40℃~50℃ for 10h.
[0047] After the reaction is complete, add 30mL of ethyl acetate, then wash with water (20mL*3), and then undergo rotary evaporation (evaporate at 40°C at a speed of 150 rpm); the residue after evaporation is separated by silica gel column, specifically For: use dichloromethane:methanol=9:1 (volume ratio) mobile phase to separate through a silica gel column (200-300 mesh) to obtain 130mg of the product. The product is 3,4-dinitrobenzoyl propargyl amide in the form of light yellow solid; the 3,4-dinitrobenzoyl propargyl amide is the precursor comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com