Methods for the production of 2-halo-4-nitroimidazole and intermediates thereof
A technology of nitroimidazole and dinitroimidazole, applied in antibacterial drugs, organic chemistry, etc., can solve the problem of low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
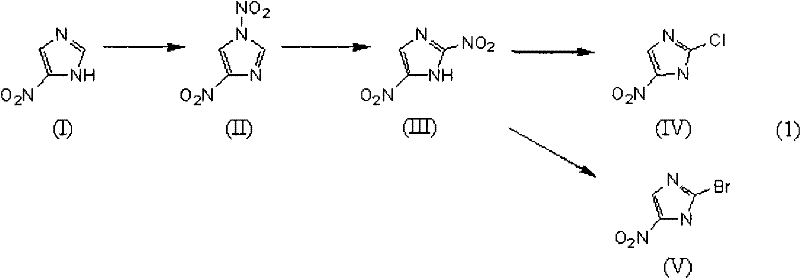
[0213]The method for preparing 2-halo-4-nitroimidazole in this embodiment is a method for preparing 2-halo-4-nitroimidazole comprising the following steps: (i) subjecting 4-nitroimidazole to a nitration reaction to obtain 1,4-dinitroimidazole; (ii) without separation or drying treatment, 1,4-dinitroimidazole dissolved in solvent or wetted with solvent is subjected to thermal reformation reaction to obtain 2,4-dinitroimidazole imidazole; and (iii) halogenating the 2,4-dinitroimidazole wetted with the solvent of the thermal reforming reaction with a halogenating agent.
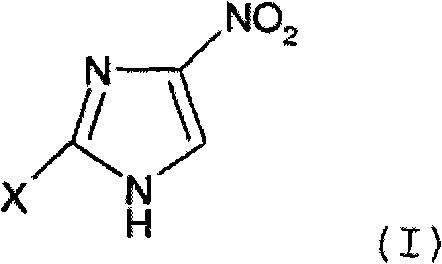
[0214] The production method of the present embodiment is a method according to a reaction scheme represented by the following formula (2).
[0215]
[0216] (wherein, X represents a halogen atom.)
[0217] Herein, the halogen atom represented by X means any one of a fluorine atom, a chlorine atom, a bromine atom or an iodine atom.
[0218] Each step will be described separately below.
[0219] [step (i)] ...
Embodiment 1
[0299] step 1
[0300] In a first vessel, 1.00 equivalents of 4-nitroimidazole (solid) was mixed with 2.01 equivalents of acetic anhydride and 5.83 equivalents of acetic acid. The mixture was cooled to 15°C. To this suspension was added 1.5 equivalents of nitric acid. The reaction is slightly exothermic and it must be ensured that the temperature of the reaction mixture does not exceed 15°C. After adding nitric acid, the reaction mixture was heated to 23 to 25°C and stirred for 3 hours. After stirring for about 2 hours, a yellow-orange solution appeared.
[0301] In a second container, combine 69.72 equivalents of water and 7.35 equivalents of sodium chloride and stir at ambient temperature until all of the sodium chloride is dissolved. 19.17 equivalents of dichloromethane were added. Then, the biphasic mixture was cooled to 15°C.
[0302] The reaction mixture in the first vessel was added to the stirred vessel (second vessel). The quenching treatment is weakly exotherm...
Embodiment 2-1
[0332] Preparation of 1,4-dinitroimidazole and 2,4-dinitroimidazole
[0333] After putting 20 g of 4-nitroimidazole into the flask, 36.4 mL of acetic acid was added thereto, and while keeping the temperature of the reaction solution below 20° C., 14.6 mL of 97% by mass nitric acid was added dropwise thereto. After completion of the dropwise addition of nitric acid, 36.4 mL of acetic anhydride was added dropwise thereto, and after raising the temperature of the reaction solution to 25° C., the resulting mixture was allowed to react for 3 hours. After the reaction was completed, the reaction solution was added to 100 g of ice water. Extraction was performed three times with 100 mL of dichloromethane, and the organic layer was washed twice with 100 mL of saturated aqueous sodium bicarbonate solution. The organic layer was separated, and 30 g of magnesium sulfate was added thereto for dehydration, thereby preparing a dichloromethane solution of 1,4-dinitroimidazole. Subsequently...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com