Preparation method and application of tanshinone II A solid dispersion pellets
A solid dispersion, tanshinone technology, applied in the field of medicine, can solve the problems of heat-sensitive drug stability, unfavorable reproducibility and controllability, loss of raw materials and excipients, etc. Simple and feasible, the effect of improving solubility and dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
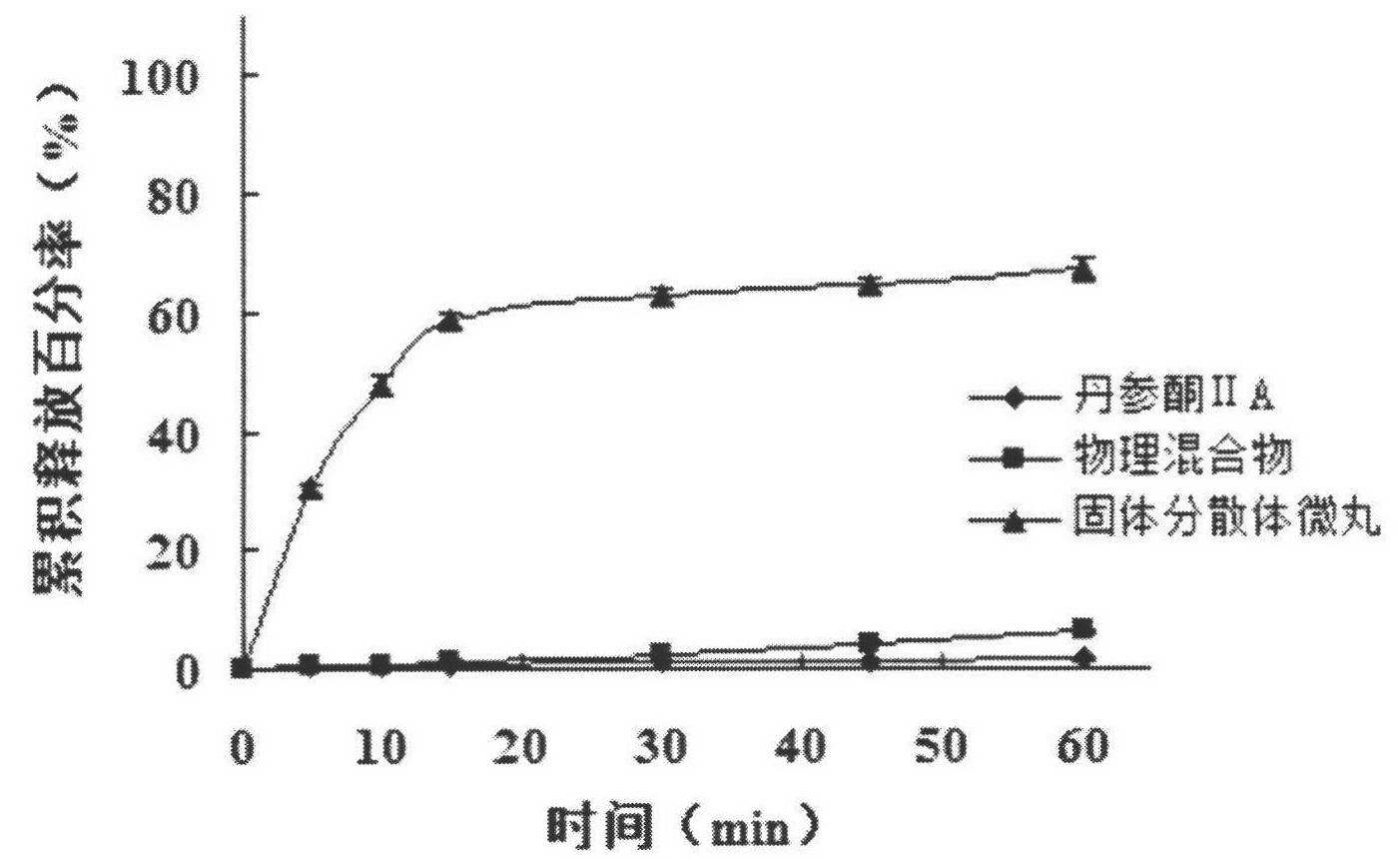
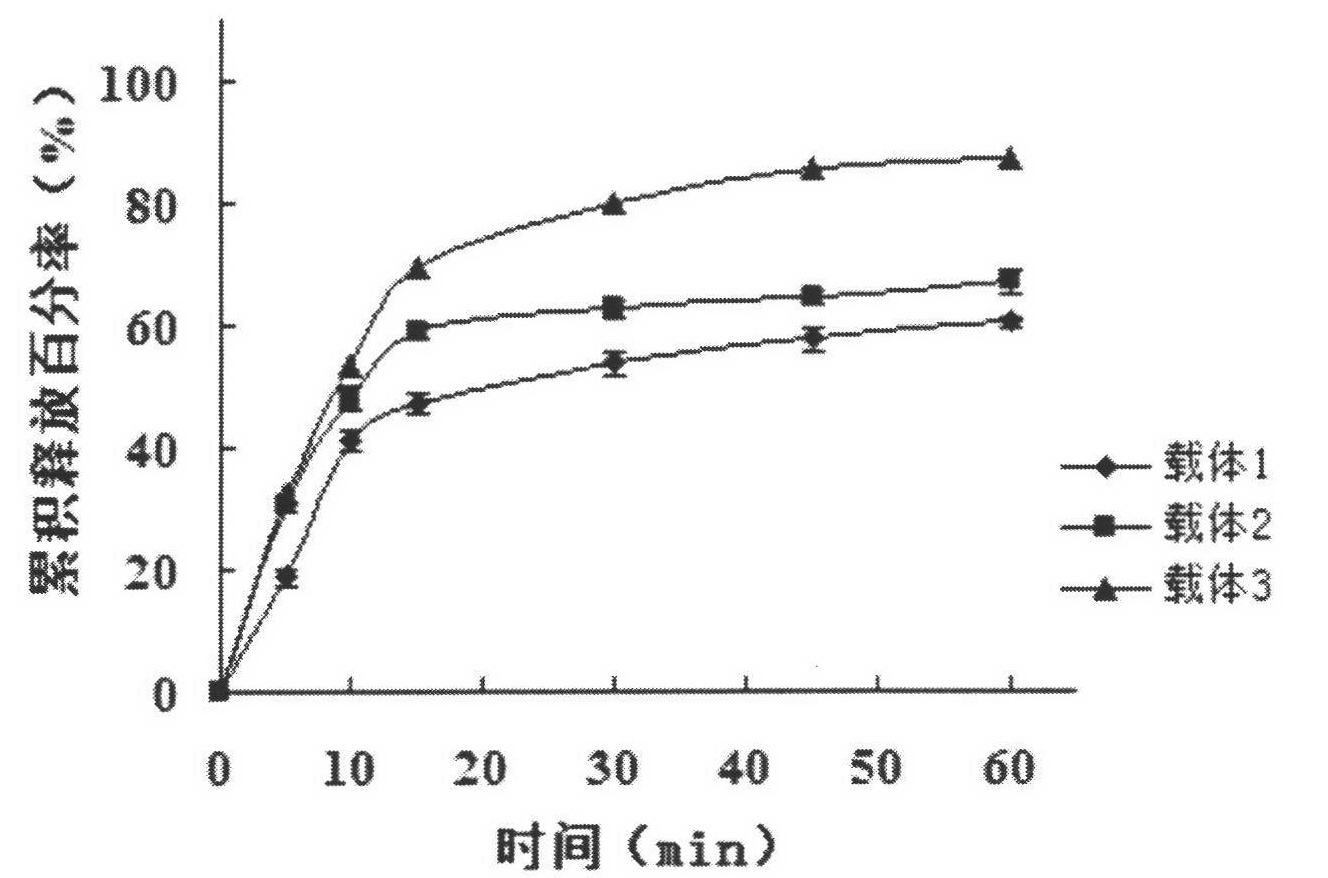
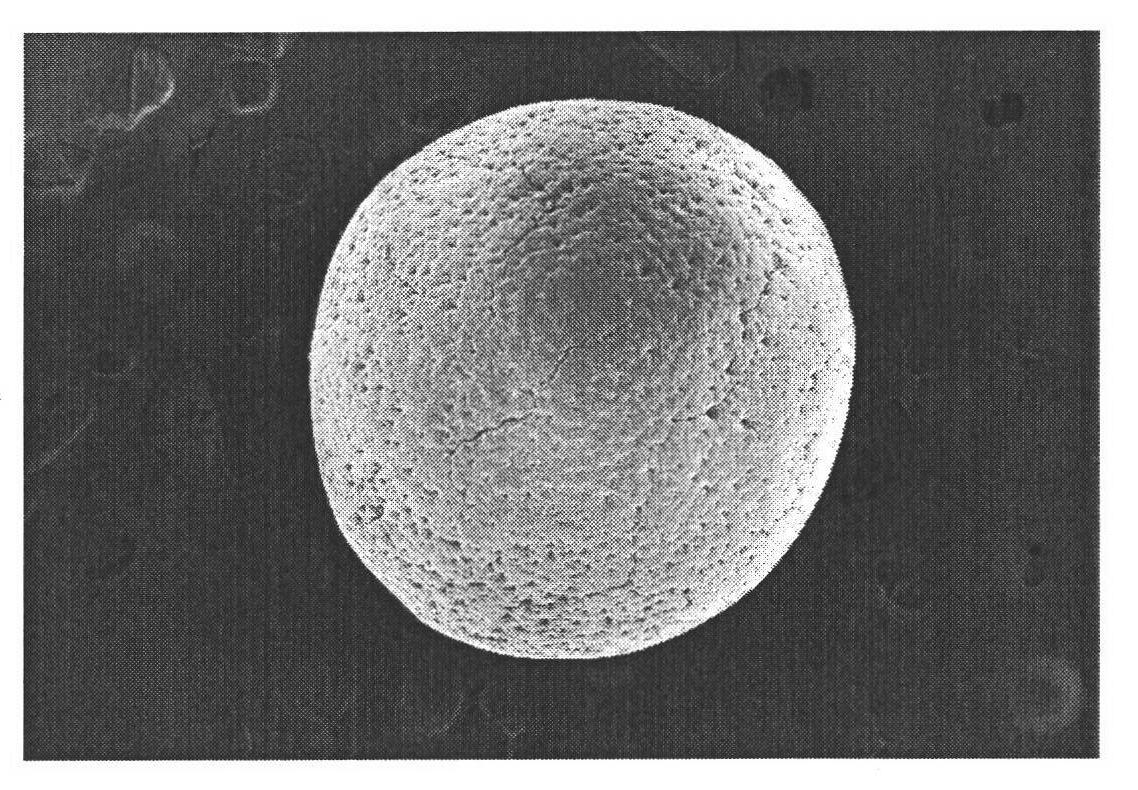
Image
Examples
Embodiment 1
[0030] Weigh 1.3g of Tanshinone IIA and 6.5g of PVPK30 and dissolve them in 95ml of ethanol, stir until completely dissolved, and wait for the medicine to be applied. Weigh 50g of blank ball cores and add them to the hopper, turn on the fan and heating device, preheat the balls in a fluidized state for a period of time, and start adding medicine. The preparation process parameters of the fluidized bed one-step molding are coating temperature 45°C, blast frequency 30Hz, spray gun spray pressure 0.3MPa, liquid spray rate 5ml / min. After the drug application is completed, the drug-loaded pellets are kept boiling for a period of time in a fluidized state to obtain the final product.
[0031] Fill the solid dispersion pellets into capsules to obtain tanshinone IIA quick-release pellets capsules.
Embodiment 2
[0033] Weigh 1.3g of tanshinone IIA and 10g of PEG4000 and PEG6000 and dissolve them in 75ml of ethanol and 25ml of acetone respectively, heat the acetone solution of polyethylene glycol to dissolve, then add it dropwise into the ethanol solution of tanshinone IIA, stir until completely dissolved, and then apply the medicine. Weigh 50g of blank ball cores and add them to the hopper, turn on the fan and heating device, preheat the balls in a fluidized state for a period of time, and start adding medicine. The preparation process parameters of the fluidized bed one-step molding are coating temperature 35°C, blast frequency 27Hz, spray gun spray pressure 0.25MPa, liquid spray rate 4ml / min. After the drug application is completed, the drug-loaded pellets are kept boiling for a period of time in a fluidized state to obtain the final product.
[0034] The solid dispersion pellets are uniformly mixed with microcrystalline cellulose, pregelatinized starch and magnesium stearate, and t...
Embodiment 3
[0036]Weigh 1.3g of tanshinone IIA and 7.5g of citric acid and dissolve them in 60ml of ethyl acetate and 10ml of 95% ethanol respectively, add the citric acid ethanol solution dropwise into the tanshinone IIA ethyl acetate solution, stir until completely dissolved, and wait for the medicine . Weigh 50g of blank ball cores and add them to the hopper, turn on the fan and heating device, preheat the balls in a fluidized state for a period of time, and start adding medicine. The preparation process parameters of the fluidized bed one-step molding are coating temperature 32°C, blast frequency 25Hz, spray gun spray pressure 0.3MPa, liquid spray rate 3ml / min. After the drug application is completed, the drug-loaded pellets are kept boiling for a period of time in a fluidized state to obtain the final product.
[0037] The solid dispersion pellets are coated with an aqueous dispersion of ethyl cellulose, and the obtained sustained-release pellets are filled in capsules to obtain tan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com