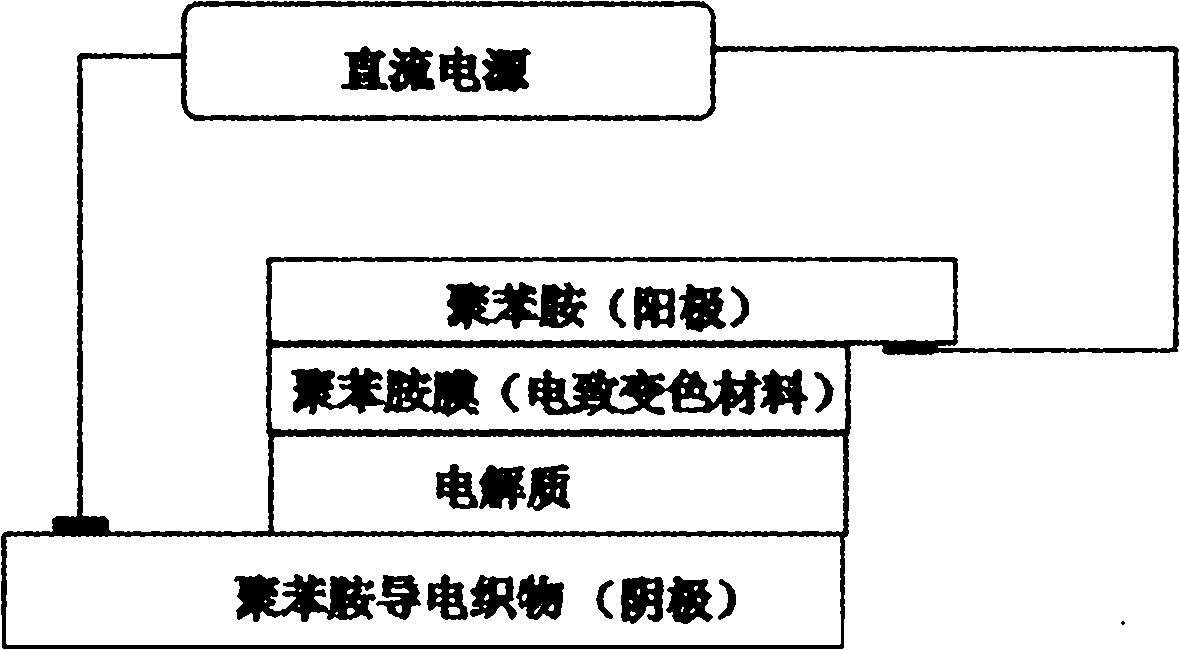
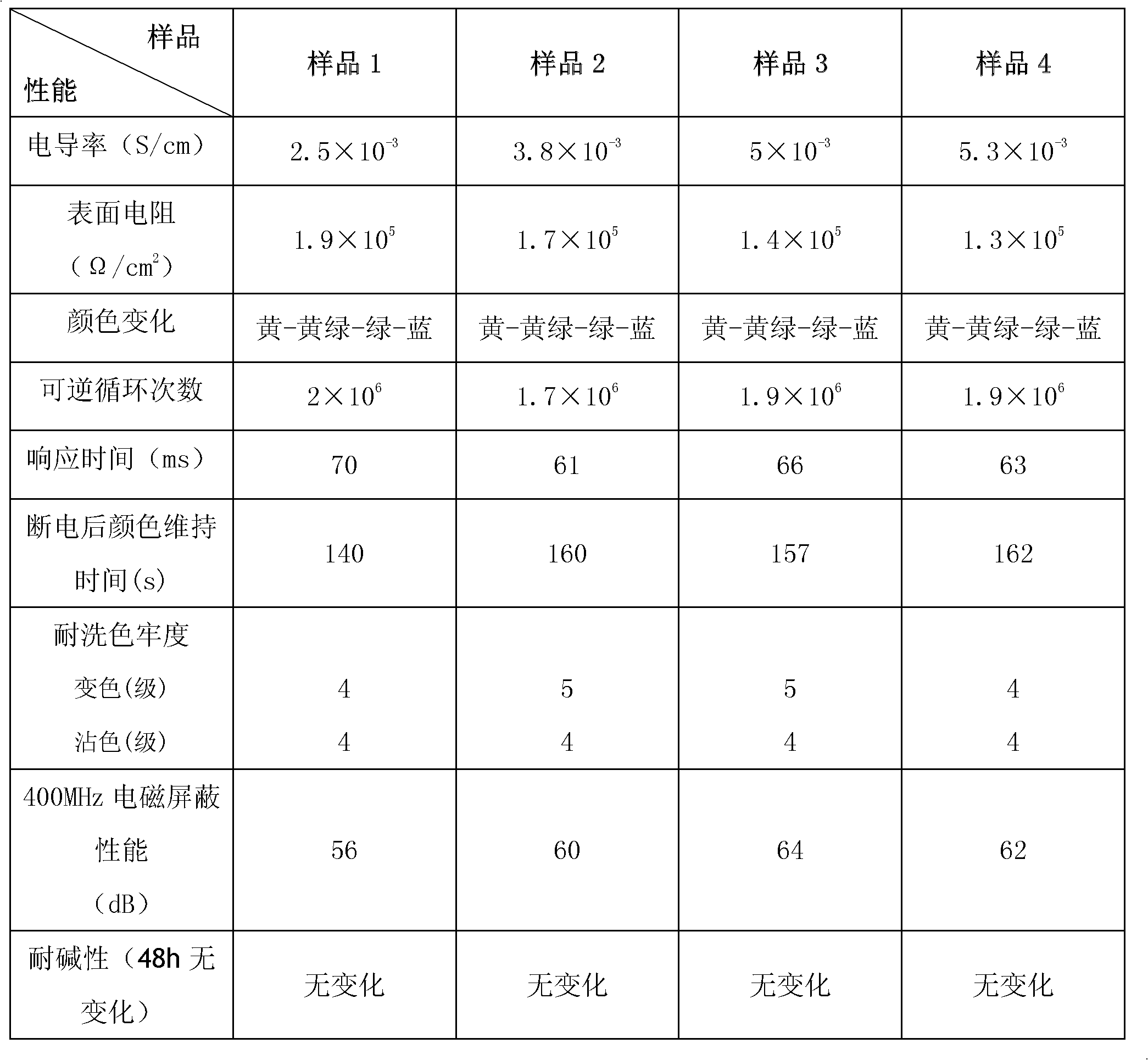
Polymer electrochromic fabric and preparation method thereof
A technology of electrochromic and electrochromic devices, which is applied in the fields of textiles, papermaking, optics, instruments, etc., can solve the problems of difficult polyaniline film with large area, poor solubility of polyaniline, low conductivity, etc., and achieve simple preparation method, Excellent and durable electrical conductivity, uniform film thickness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Step 1. Dissolve 1 mL of 0.1 mol / L aniline and the same amount of o-aminobenzenesulfonic acid as aniline in 1 mL of 0.1 mol / L H 2 SO 4 In solution, get solution A;
[0031] Step 2. Put a certain amount (8mg) of fiber into 4mL of 0.1mol / L H 2 SO 4 , And then add 4mL cyclohexane to the solution, shake in ultrasonic for 12h to obtain solution B;
[0032] Step 3. Soak B in solution A, put it into the reaction tank, and ultrasonically disperse for 1 hour.
[0033] Step 4. The electrode is polished and chemically treated, and cyclic voltammetry is scanned in a potential range of -0.10~0.95V in 50mL of 0.5mol / L HCl solution until a stable cyclic voltammogram is obtained, and then Insert the polarized electrode into the reaction cell for 10 minutes, take out the electrode and soak it in anhydrous cyclohexane, rinse it with hydrochloric acid, acetone, and water in turn, and use N 2 Blow dry and dry at 40°C for 1 hour.
[0034] Step 5. Slowly add 4mL 0.1mol / L(NH 4 ) 2 S 2 O 8 , React for...
Embodiment 2
[0037] Step 1. Dissolve 5 mL of 0.2mol / L aniline and the same amount of o-aminobenzoic acid as aniline in 2 mL of 0.5mol / L p-toluenesulfonic acid solution to obtain solution A;
[0038] Step 2. Put a certain amount (14mg) of fiber into 12mL of 0.4mol / L p-toluenesulfonic acid, and then add 6mL of cyclohexane to the solution, and vibrate in ultrasonic for 16h to obtain solution B;
[0039] Step 3. Soak B in the A solution, put it in the reaction tank, and ultrasonically disperse for 2h.
[0040] Step 4. The electrode is polished and chemically treated, and the electrode is exposed to 60mL of 0.55mol / L H 2 SO 4 In the solution, scan the cyclic voltammetry in the potential range of -0.10~0.95V until a stable cyclic voltammogram is obtained, then insert the polarized electrode into the reaction cell for 15 minutes, and take out the electrode soaked in anhydrous cyclohexane. Wash with hydrochloric acid, acetone, and water in turn, and use N 2 Blow dry and dry at 45°C for 1.5h.
[0041] Step...
Embodiment 3
[0044] Step 1. Dissolve 13mL of 0.34mol / L aniline and N-(4-sulfophenyl)aniline equivalent to aniline in 8mL of 0.55mol / L camphorsulfonic acid solution to obtain solution A;
[0045] Step 2. Put a certain amount (12 mg) of fibers into 10 mL of 0.4 mol / L polyvinyl chloride sulfonic acid, and then add 8 mL of cyclohexane to the solution, and vibrate in ultrasonic for 18 hours to obtain solution B;
[0046] Step 3. Soak B in solution A, put it in the reaction tank, and ultrasonically disperse for 2.5h.
[0047] Step 4. The electrode is polished and chemically treated, at 0.7mol / L of H 3 PO 4 In the solution, carry out cyclic voltammetry scanning in the potential range of -0.10~0.95V until a stable cyclic voltammogram is obtained, then insert the polarized electrode into the reaction cell for 25 minutes, and take out the electrode and soak it in anhydrous cyclohexane. Wash with hydrochloric acid, acetone, and water in turn, and use N 2 Blow dry and dry at 55°C for 2.5h.
[0048] Step 5. Sl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com