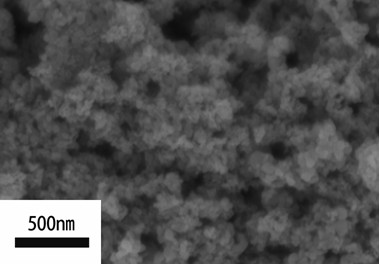
Method for preparing calcium titanate nanoparticles
A technology of nanoparticles and calcium titanate, which is applied in the field of preparation of calcium titanate nanoparticles, can solve the problem of particle size reduction without calcium titanate materials, and achieve the effect of low cost and easy production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0021] 1) Dissolve tetrabutyl titanate in ethylene glycol methyl ether to adjust the Ti in the solution 4+ The ion concentration is 0.5mol / L;
[0022] 2) Under stirring, add 1.5ml of ammonia solution with a mass concentration of 30% to the tetrabutyl titanate ethylene glycol methyl ether solution prepared in 1), precipitate, filter, and wash with deionized water for 6 times to obtain titanium Co-precipitation of oxyhydroxides;
[0023] 3) Dissolve calcium nitrate in deionized water to adjust the Ca in the solution 2+ The ion concentration is 1.5mol / L;
[0024] 4) Potassium hydroxide is dissolved in deionized water, and the configuration concentration is an aqueous potassium hydroxide solution of 2mol / L;
[0025] 5) Put 6g glucose at 180 o C hydrothermal reaction 10h, obtains glucose hydrothermal decomposition solution;
[0026] 6) Add titanium oxyhydroxide co-precipitation, calcium nitrate aqueous solution, glucose hydrothermal decomposition solution 20ml and potassium hy...
example 2
[0029] 1) Dissolve tetrabutyl titanate in ethylene glycol methyl ether to adjust the Ti in the solution 4+ The ion concentration is 1mol / L;
[0030] 2) Under stirring, add 1.5ml of ammonia solution with a mass concentration of 30% to the tetrabutyl titanate ethylene glycol methyl ether solution prepared in 1), precipitate, filter, and wash with deionized water for 6 times to obtain titanium Co-precipitation of oxyhydroxides;
[0031] 3) Dissolve calcium nitrate in deionized water to adjust the Ca in the solution 2+ The ion concentration is 2mol / L;
[0032] 4) Potassium hydroxide is dissolved in deionized water, and the configuration concentration is an aqueous potassium hydroxide solution of 2 mol / L;
[0033] 5) Put 6g glucose at 180 o C hydrothermal reaction 10h, obtains glucose hydrothermal decomposition solution;
[0034] 6) Add titanium oxyhydroxide co-precipitation, calcium nitrate aqueous solution, glucose hydrothermal decomposition solution 20ml and potassium hydro...
example 3
[0037] 1) Dissolve tetrabutyl titanate in ethylene glycol methyl ether to adjust the Ti in the solution 4+ The ion concentration is 0.6mol / L;
[0038] 2) Under stirring, add 1.0ml of ammonia solution with a mass concentration of 30% to the tetrabutyl titanate ethylene glycol methyl ether solution prepared in 1), precipitate, filter, and wash with deionized water for 6 times to obtain titanium Co-precipitation of oxyhydroxides;
[0039] 3) Dissolve calcium nitrate in deionized water to adjust the Ca in the solution 2+ The ion concentration is 2.4mol / L;
[0040] 4) Potassium hydroxide is dissolved in deionized water, and the configuration concentration is an aqueous potassium hydroxide solution of 2 mol / L solution;
[0041] 5) Put 5g glucose at 160 o C hydrothermal reaction 12h, obtains glucose hydrothermal decomposition solution;
[0042] 6) Add titanium oxyhydroxide co-precipitation, calcium nitrate aqueous solution, glucose hydrothermal decomposition solution 20ml and po...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com