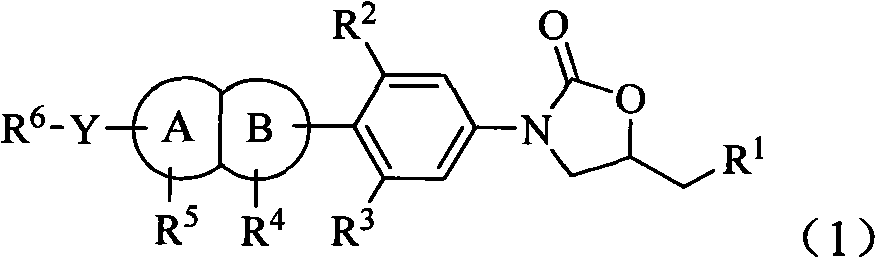
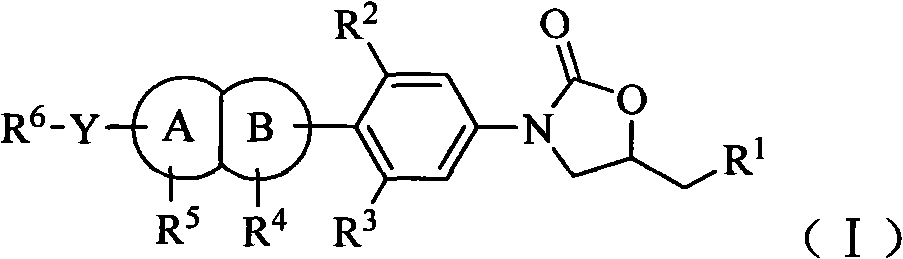
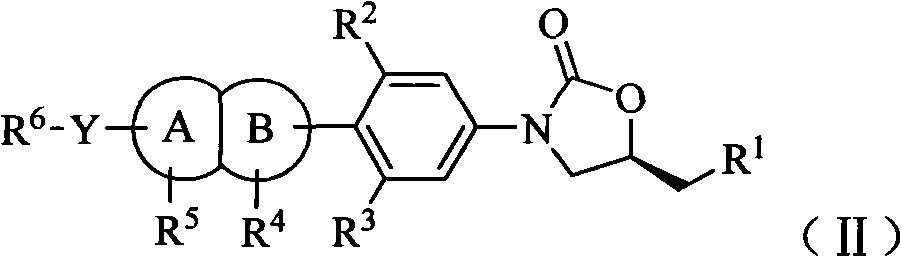
Oxazolidinone antibiotic containing parallel rings
An alkyl and alkylamine-based technology, applied in the field of medicine, can solve the problems of linezolid drug resistance, single oxazolidinone antibiotics, and inability to meet clinical medication requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0161] Example 1 (S)-N-[[3-[3-fluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzene Base]-2-oxo-5-oxazole Preparation of alkyl] methyl] acetamide
[0162]
[0163] Add 30 mL of 1,4-dioxane to the dry reaction flask, (S)N-[[3-(3-fluoro-4-bromophenyl)-2-oxo-5-oxazolidinyl ] methyl] acetamide 3.31g (10mmol), bis-valeryl diborane 2.54g (10mmol), potassium acetate 0.98g (10mmol), feed argon into the reaction flask, then add Pd (PPh 3 ) 2 Cl 2 0.3g, continue to feed argon into the reaction solution, stir the reaction overnight at 90°C, cool to room temperature, filter with diatomaceous earth, extract with ethyl acetate and brine, dry and concentrate the organic layer with anhydrous sodium sulfate, and precipitate a gray solid , 3.22 g of the product was obtained with a yield of 85.2%. ((S)-N-[[3-[3-fluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2 used in the following examples -base) phenyl]-2-oxo-5-oxazolidinyl] methyl] the preparation of acetamide is prepare...
Embodiment 2
[0164] Example 2N-[[(5S)-3-[4-[1-[(1H-1,2,3-triazol-5-yl)methanamine]-2,3-two Hydrogen-1H-inden-5-yl]-3-fluorophenyl]-2- Preparation of oxo-5-oxazolidinyl]methyl]acetamide hydrochloride (compound 1 hydrochloride)
[0165]
[0166] (1) Preparation of 5-bromo-N-(2-propynyl)-2,3-dihydro-1H-inden-1-amine
[0167]
[0168] Dissolve propargylamine (6.6g, 0.12mol) and 5-bromo-1-indanone (22.9g, 0.109mmol) in 1,2-dichloroethane (300mL), and add to it under an ice-water bath Add NaBH(OAc) 3 (43.3g, 203mmol), after the addition, the mixture was stirred and reacted at room temperature for 40 hours, the organic phase was washed with water (50mL×2) and NaCl (150mL×2) successively, dried over anhydrous magnesium sulfate, and the organic phase was used directly without purification in the next step.
[0169] (2) Preparation of tert-butyl 5-bromo-2,3-dihydro-1H-inden-1-yl (2-propynyl) carbamate
[0170]
[0171] 5-Bromo-N-(2-propynyl)-2,3-dihydro-1H-inden-1-amine (8.9 g, 0.0...
Embodiment 3
[0186] Example 3N-[[(5S)-3-[4-[1-[2-(1H-1,2,3-triazol-5-yl)ethyl]indoline-5-yl]-3 -Fluorophenyl]-2-oxo - Preparation of 5-oxazolidinyl] methyl] acetamide (compound 2)
[0187]
[0188] (1) Preparation of 3-butynyl methanesulfonate
[0189]
[0190] In a 250mL reaction flask, dissolve 0.500g (7.13mmol) of 3-butyn-1-ol and 2.17g (21.4mmol) of triethylamine with 90mL of dichloromethane, and slowly add methanesulfonyl chloride 1.06 g (9.23mmol), added dropwise for about 30 minutes, stirred at room temperature overnight (16 hours) after removing the cooling bath, the organic phase was washed with 1M HCl (aqueous solution) and sodium chloride successively, then dried with anhydrous magnesium sulfate, and filtered After the solvent was removed under reduced pressure, 1.02 g of oily 3-butynyl methanesulfonate was obtained, with a yield of 96%.
[0191] (2) Preparation of 5-bromo-1-(3-butynyl)indoline
[0192]
[0193] Add Na to a toluene solution (40mL) of 4.95g (25mmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com