Engineering bacteria expressing active peptides and method of preparing mixed polypeptide
A technology of engineering bacteria and active peptides, applied in the field of bioengineering, can solve the problems of simultaneous expression that have not been reported, and achieve the effect of increasing the ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Two kinds of lactating blood pressure reduction peptides CEI-12 and CEI-7 series DNA design and synthesis
[0020] A. Two kinds of milk-derived blood pressure-lowering peptides CEI-12 and CEI-7 series polypeptide sequence design
[0021] The CEI-12 (SEQ ID NO3) and CEI-7 (SEQ ID NO4) involved in the invention involved in the present invention respectively correspond to the residual sequences of cowytan α-casein 23-34 and 177 of cowl β-casein.-183 residue sequence.The N-segment amino acid residues of CEI-12 and CEI-7 are LYS and ARG, respectively, and the enzyme sect sites that are also anotinase.The n segment adds ASP-ASP-ASP-ASP-LYS sequence, and the sequence is shown in SEQ ID NO2.
[0022] B. Two kinds of milk-derived blood pressure peptide CEI-12 and CEI-7 series polypeptide genetic design
[0023] SEQ ID NO2 (SEQ ID NO2) depending on E. coli ( Escherichia color ) BL-21 strain codons prefer sex transformation into nucleotide sequences and add TAATAATAA sequen...
Embodiment 2
[0024] Example 2: The construction of the expression carrier PUC18-CEI-12,7 and the construction of E. coli BL21-MF3A3 engineering bacteria
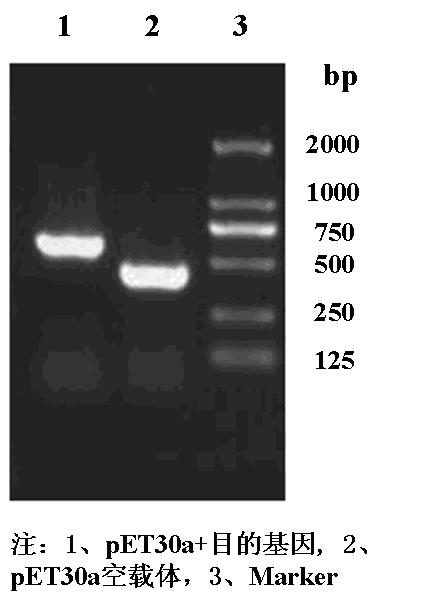
[0025] A. The nucleotide sequence designed by the Example 1 SEQ ID NO1 is synthesized by Shanghai Xuguan Biotechnology Development Co., Ltd., cloned to PUC18 KPN Ⅰ and Bam Hi, named this restructuring carrier to PUC18-CEI-12,7, and transfer to E. coli ( Escherichia color ) DH5α (purchased from Novagen).
[0026] b. Extraction of E. coli DH5α containing PUC18-CEI-12,7 to extract plasmid KPN Ⅰ and Bam Hi (purchased from Takara Company) dual enzyme cutting and recycling about 200bp.
[0027] C. For the expression carrier PET-30A (purchased from Novagen) KPN Ⅰ and Bam Hi (purchased from Takara Company) dual enzyme cutting, recycling large segments.
[0028]D. The recycling clip of the viscous end of the step B is connected to the large segments recovered in the step C, transferred to the DH5α strain, and the PCR of conventional colonies is used ...
Embodiment 3
[0031] Example 3: The expression and detection of two kinds of lactating blood pressure reduction peptides CEI-12 and CEI-7 series polypeptides
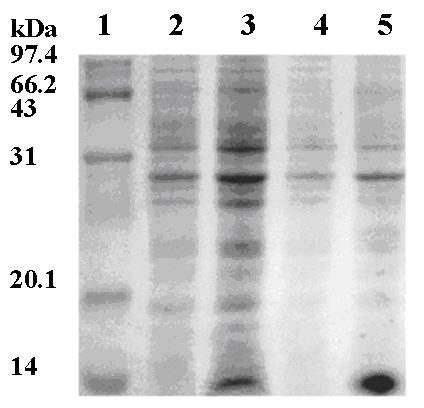
[0032] The LB liquid medium (50 μg / ml) LB liquid medium obtained from E. coli BL21-MF3A3 obtained by example 2 was inoculated at a volume ratio of 1%in volume ratio at a volume ratio of 1%., NaCl 10g / L, pH7.0), 37 ° C, 200R · min -1 When the constant temperature shake bed is cultivated to 1.5, adding the isopropyte-β-D-sulfur sulfurcoside (IPTG) to the final concentration 1mmol·L –1 , Live 12 h, 5000r · min -1 Collect the bacteria for centrifugation, repeatedly frozen the bacteria 3 times, and perform ultrasonic crushing (300W, 2S / 2min, 2min), 8000R · min, 8000R · min -1 Corporation of centrifugal precipitation, dissolved in the 8ml upper column buffer solution, and purified the reorganized protein marked by the activated NI-column (purchased from Novagen) to perform SDS-PAGE electrophoresis, such as figure 2 Show.Electric swings indicat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com