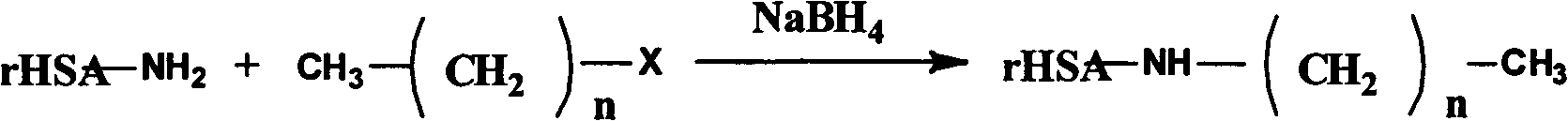Alkylating recombinant human serum albumin and preparation and application of medicinal composition thereof
A human serum albumin and drug technology, applied in the direction of inactive components of polymer compounds, etc., can solve the problems of source limitation, no literature and patent reports on nano micelles, etc., to achieve high drug loading and avoid potential virus transmission. Dangerous, high encapsulation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
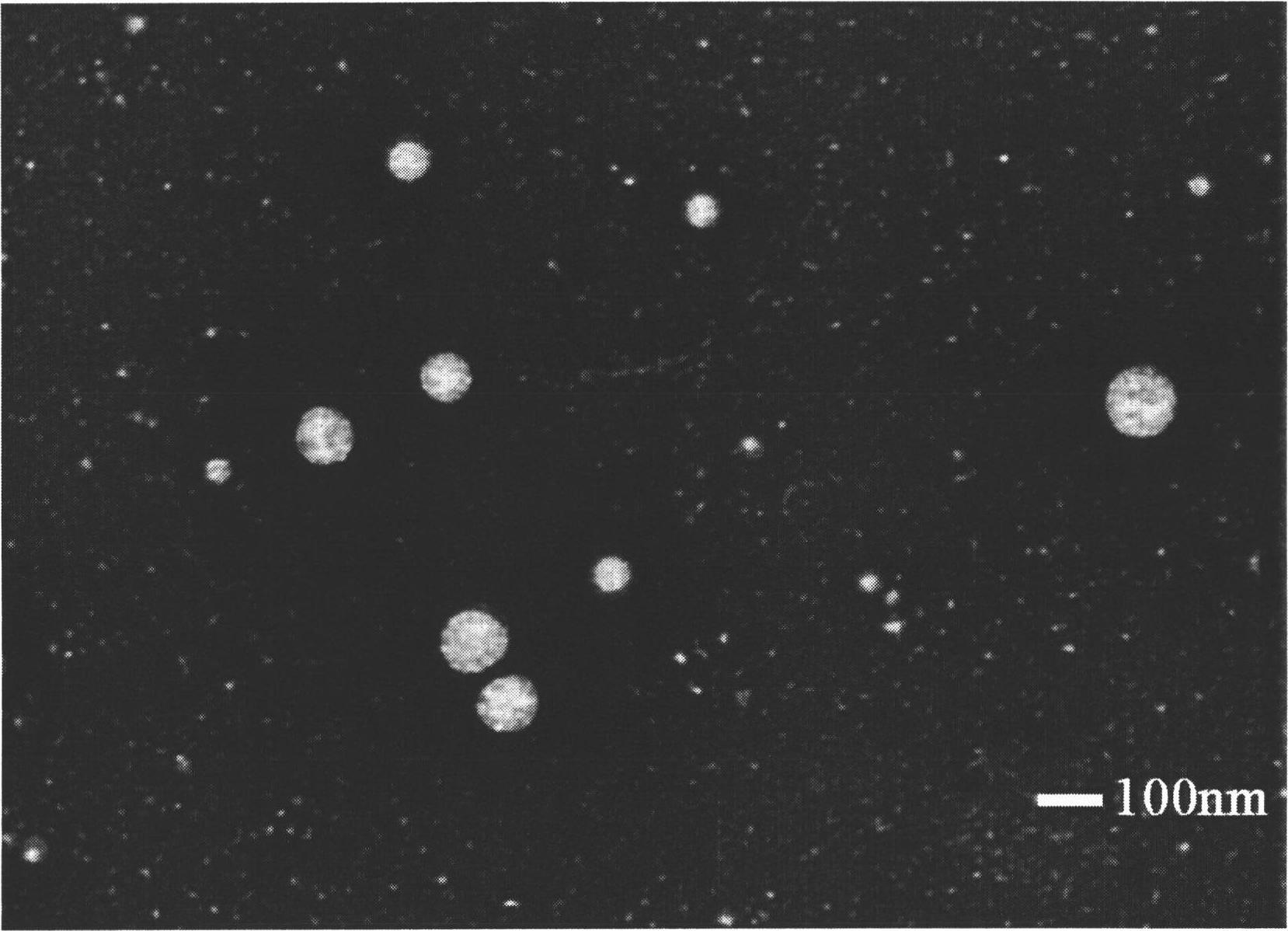
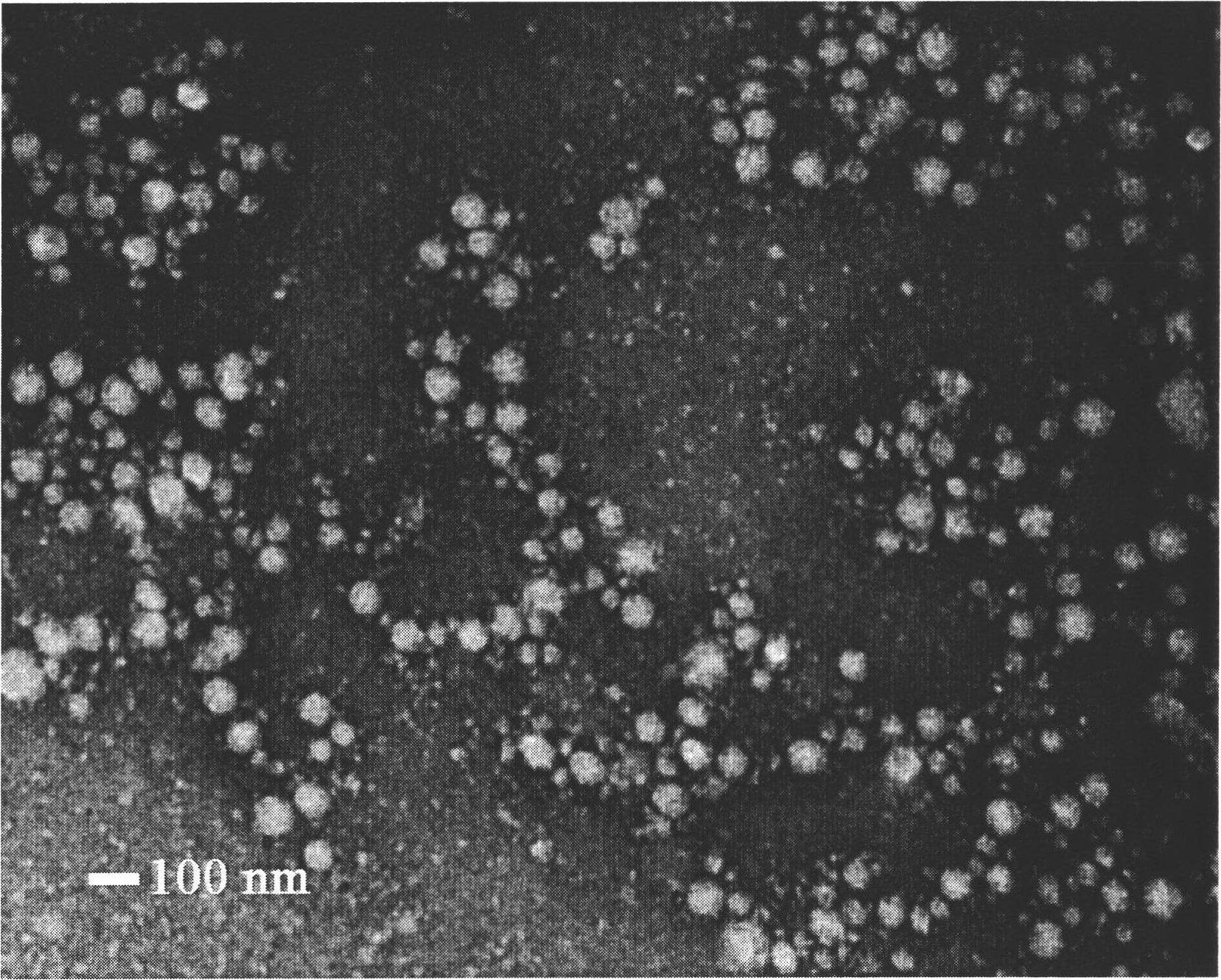
Image
Examples
Embodiment 1
[0058] Take 1g of rHSA, dissolve it in water, add 0.5292g of n-butyraldehyde, react at 30°C for 0.5h, then add 10% NaBH 4 or NaCNBH 3 The solution was stirred for 12 hours, adjusted to pH 7.0 with 2 mol / L HCl solution, dialyzed in 10 mM phosphate buffer solution (pH 7.4) for 3 days (MWCO=12000-14000), freeze-dried, and obtained. The results of elemental analysis showed that 56 n-butyl groups were introduced into one rHSA molecular chain.
Embodiment 2
[0060] Take 1g of rHSA, dissolve it in water, add 0.9408g of n-octanal, react at room temperature for 0.5h, add NaBH with a concentration of 10% 4 or NaCNBH 3 The solution was stirred for 12 hours, adjusted to pH 7.0 with 2 mol / L HCl solution, dialyzed in 10 mM phosphate buffer solution (pH 7.4) for 3 days (MWCO=12000-14000), freeze-dried, and obtained. The results of elemental analysis showed that 42 n-octyl groups were introduced into one rHSA molecular chain.
Embodiment 3
[0062] Take 1g of rHSA, dissolve it in water, add 0.2867g of n-decyl aldehyde, react at room temperature for 0.5h, then add 10% NaBH 4 or NaCNBH 3 The solution was stirred for 12 hours, adjusted to pH 7.0 with 2 mol / L HCl solution, dialyzed in 10 mM phosphate buffer solution (pH 7.4) for 3 days (MWCO=12000-14000), and freeze-dried to obtain the obtained product. The results of elemental analysis showed that 9 n-decyl groups were introduced into one rHSA molecular chain.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com