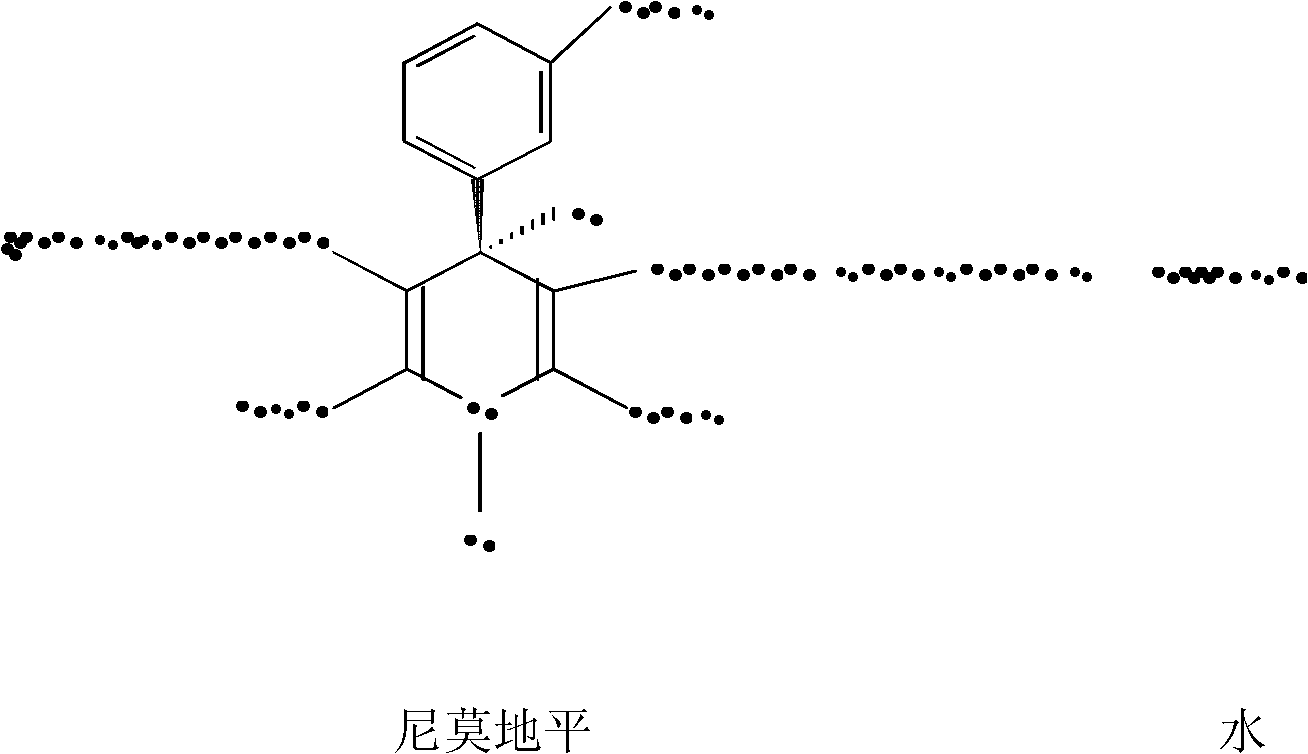
Preparation method of nimodipine
A nimodipine, reaction technology, applied in the field of preparation of nimodipine, can solve the problem of low process yield, achieve high yield, reduce raw material cost, and achieve good quality effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
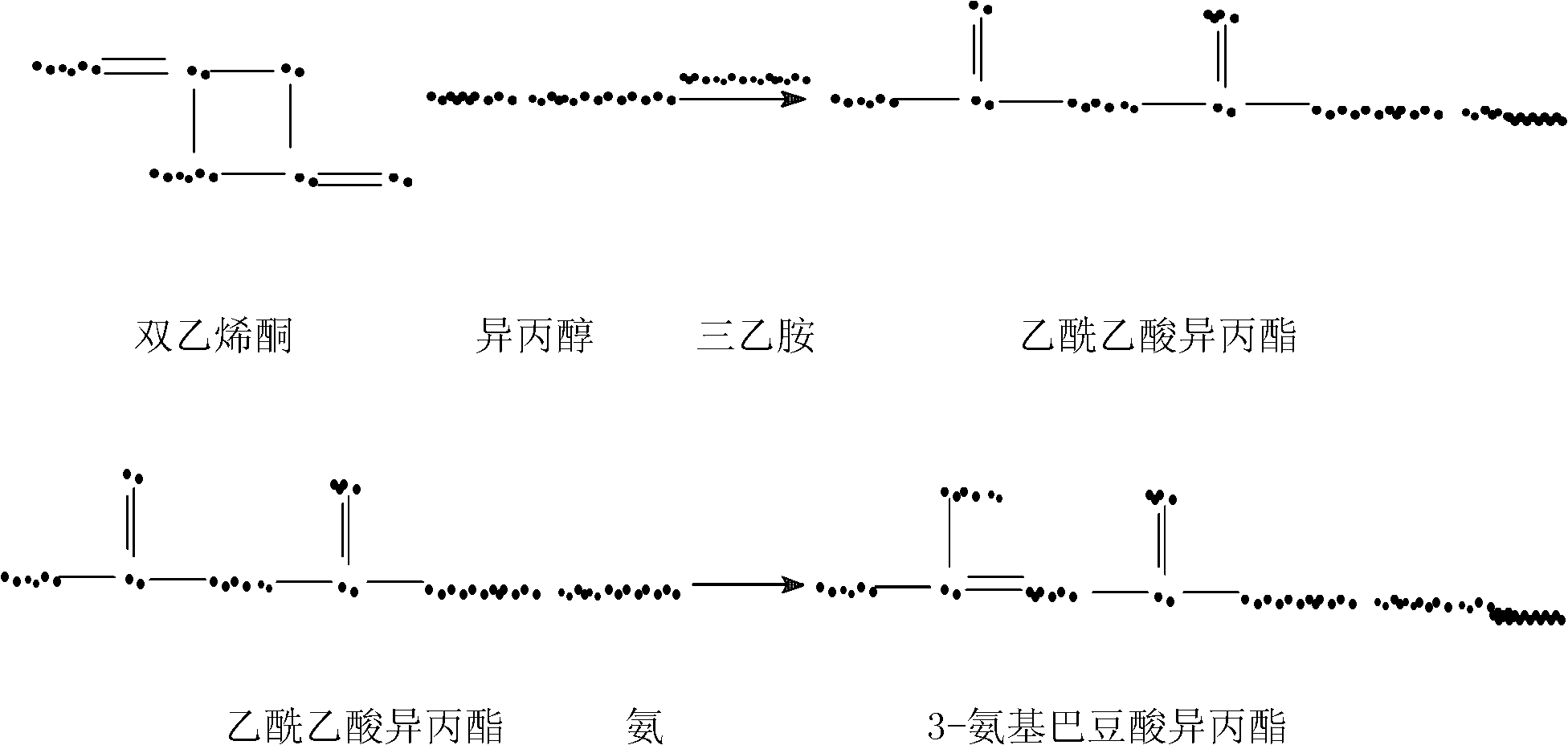
[0020] Put 64kg of isopropanol and 0.4kg of triethylamine into the reaction tank, raise the temperature to 75°C, add 90kg of diketene dropwise for 1 hour, control the temperature at 80±2°C, and then keep the reaction at 90°C for 3 hours. Cool down to 0°C, pass ammonia for 5 hours, the amount of ammonia passed is 18kg, and stand for 3 hours to keep warm for reaction. Add 5.7 kg of anhydrous calcium chloride, stir to dissolve for 30 minutes, and stand still for 3 hours to separate water. Distilled under reduced pressure to obtain 125 kg of colorless oily isopropyl 3-aminocrotonate with a yield of 81.5% and a content of 98.8%.
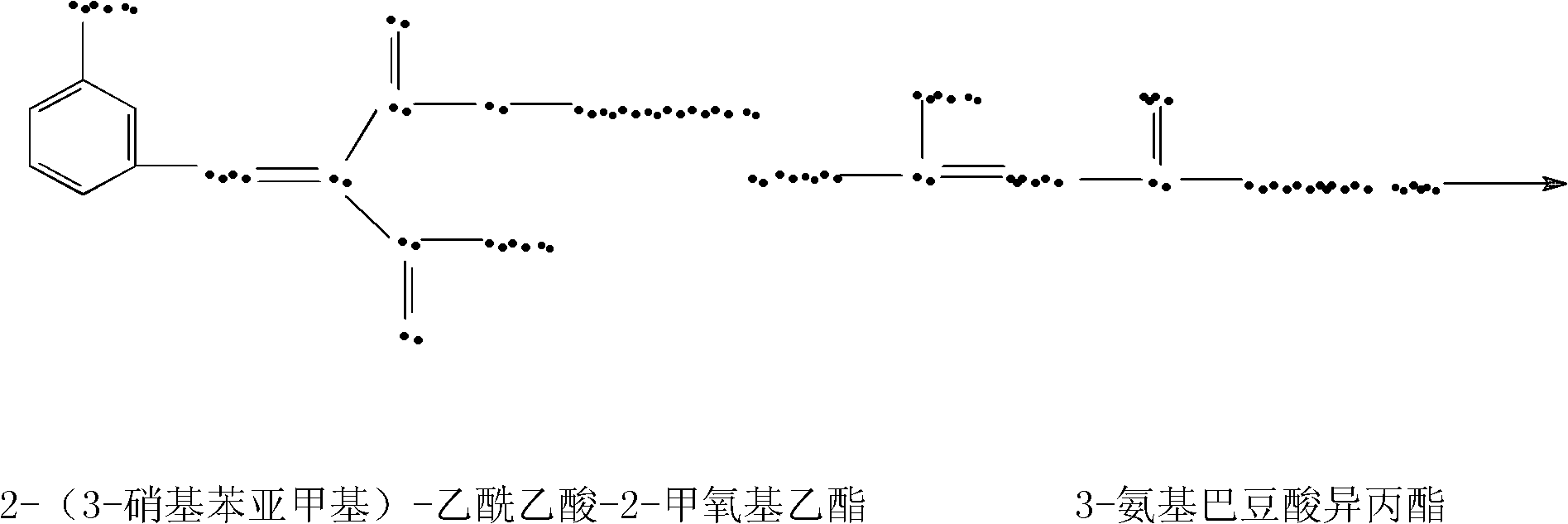
[0021] Put 52kg of isopropyl 3-aminocrotonate, 100kg of 2-(3-nitrobenzylidene)-2-methoxyethyl acetoacetate and 105kg of methanol into the reaction tank, raise the temperature to 30°C, and keep the temperature for 1 hour. The reaction was carried out under reflux for 1 hour, and the methanol was distilled under reduced pressure until no more distillation ...
Embodiment 2
[0023] Put 65kg of isopropanol and 0.5kg of triethylamine into the reaction tank, raise the temperature to 75°C, add 90kg of diketene dropwise for 2 hours, control the temperature at 80±2°C, and then keep the reaction at 95°C for 3 hours. Cool down to 10°C, pass ammonia for 5 hours, the amount of ammonia passed is 18kg, and stand for 3 hours to keep warm for reaction. Add 4.3 kg of anhydrous magnesium sulfate, stir to dissolve for 30 minutes, and stand still for 3 hours to separate water. Distilled under reduced pressure to obtain 126 kg of colorless oily isopropyl 3-aminocrotonate with a yield of 82.2% and a content of 98.4%.
[0024] Put 56kg of isopropyl 3-aminocrotonate, 100kg of 2-(3-nitrobenzylidene)-2-methoxyethyl acetoacetate and 122kg of ethanol into the reaction tank, raise the temperature to 70°C, and keep the temperature for 1 hour. The reaction was refluxed for 1 hour, and the ethanol was distilled under reduced pressure until no more distillation occurred. Afte...
Embodiment 3
[0026] Put 65kg of isopropanol and 0.6kg of triethylamine into the reaction tank, raise the temperature to 75°C, add 90kg of diketene dropwise for 1.5 hours, control the temperature at 80±2°C, and then keep the reaction at 90°C for 3 hours. Cool down to 5°C, pass ammonia for 5 hours, the amount of ammonia passed is 20kg, and stand for 3 hours to keep warm for reaction. Add 5.0 kg of sodium chloride, stir to dissolve for 30 minutes, and stand still for 3 hours to separate water. Distilled under reduced pressure to obtain 126 kg of colorless oily isopropyl 3-aminocrotonate with a yield of 82.2% and a content of 98.1%.
[0027] Put 58kg of isopropyl 3-aminocrotonate, 100kg of 2-(3-nitrobenzylidene)-2-methoxyethyl acetoacetate and 102kg of isopropanol into the reaction tank, raise the temperature to 50°C and keep it warm for 1 hour , refluxed for 1 hour, and distilled isopropanol under reduced pressure until no longer distilled. After the reaction was completed, 74 kg of isoprop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com