High-purity silymarin and preparation method thereof
A silibinin and high-purity technology, applied in the field of high-purity silibinin and its preparation, can solve problems such as difficulty in degreasing, difficulty in separation and purification, improve product quality and yield, reduce environmental pollution, and the preparation method simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
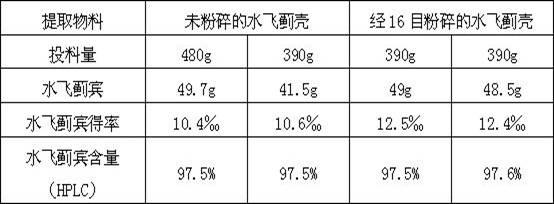
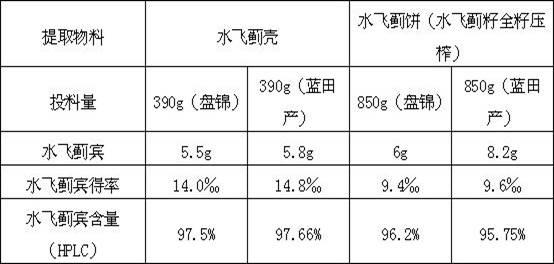
[0023] Example 1: Feed 600g of milk thistle shell powder crushed through 16 meshes, add 1020g of ethyl acetate to dissolve it, reflux to extract the total flavonoids for 2 hours, then add 780g of ethyl acetate, and reflux to extract the total flavonoids for 16 hours , combined the total flavonoids extracted twice, and collected ketones in a vacuum to obtain 82 g of total flavonoids.
[0024] 82g of total flavones were dissolved by adding purified water 5 times the mass of total flavones, stirred in a water bath and allowed to stand for 1 hour, and 246g of ethanol solution with a concentration of 50% was added to the precipitate to dissolve it to obtain 70g of silybin crude product I; Add 210g of 65% ethanol solution to the crude silibinin I to dissolve, and crystallize for 24 hours to obtain 52g of the crude silibinin II; add 520g of absolute ethanol to the crude silibinin II to dissolve, and then Microporous filtration while hot, concentrate the filtrate to about 20% of the t...
Embodiment 2
[0025] Embodiment 2: Get 600g milk thistle shell feed intake, add 1020g ethyl acetate and dissolve it, reflux extracts total flavonoids for 2 hours, then add 780g ethyl acetate, reflux extracts total flavonoids for 16 hours, merge the total flavonoids extracted twice Liquid, ketones were collected in vacuum to obtain 78g total flavonoids.
[0026] 78g total flavones were dissolved by adding purified water 5 times the total flavonoids mass, stirred in a water bath and allowed to stand for 1 hour, and the precipitate was dissolved by adding 234g of 50% ethanol solution to obtain 65g of silybin crude product I; Add 210g of ethanol solution with a concentration of 65% to the crude silibinin I to dissolve, crystallize for 24 hours to obtain 48g of the crude silibinin II; add 480g of absolute ethanol to the crude silibinin II to dissolve, and after the Microporous filtration while hot, concentrate the filtrate to about 20% of the total volume of crude silibinin II and the added abso...
Embodiment 3
[0027] Example 3: Feed 300 g of milk thistle shell powder crushed through 16 meshes, add 510 g of ethyl acetate to dissolve it, reflux to extract the total flavonoids for 2 hours, then add 390 g of ethyl acetate, and reflux to extract the total flavonoids for 16 hours , combined the total flavonoids extracted twice, and collected the ketones in a vacuum to obtain 45 g of total flavonoids.
[0028] Add purified water 3 times the mass of total flavonoids to dissolve 45g of total flavonoids, stir in a water bath and let stand for 1 hour, take the precipitate and add 135g of ethanol solution with a concentration of 50% to dissolve it, and obtain 34g of silybin crude product I; Add 102g of 65% ethanol solution to the crude silibinin I to dissolve, crystallize for 24 hours to obtain 25g of the crude silibinin II; Microporous filtration while hot, concentrate the filtrate to about 20% of the total volume of crude silibinin II and the added absolute ethanol, place at room temperature ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com