Recombinant high density lipoprotein (HDL) medicament delivery system with functions of targeted and reverse cholesterol transport (RCT) on vascular wall and application thereof
A high-density lipoprotein and drug-carrying system technology, which is applied in the field of lipid-soluble cardiovascular drug carriers, can solve the problems of endangering human health, low bioavailability, harm, etc., and achieves high drug-carrying capacity and high apolipoprotein binding rate. , the effect of improving physiological function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] Preparation process: put the prescribed amount of medicine, lecithin, and cholesterol in a vial, add an appropriate amount of chloroform, dissolve it with ultrasound, transfer it into an eggplant-shaped bottle, place it in a rotary evaporator, remove the organic phase under reduced pressure, and dry it in vacuum 2h, add an appropriate amount of hydration medium dissolved with sodium cholate, ultrasonically disperse evenly into a translucent suspension, then use a probe to ultrasonically pulverize to make it transparent and opalescent liquid, pass through a 0.22 μm filter membrane, add apolipoprotein, After dissolving, place in a refrigerator at 4°C, stir magnetically at 300rpm for 3-6h, and dialyze overnight with 2L of Tris-HCl buffer containing NaCl and sodium EDTA to obtain rHDL.
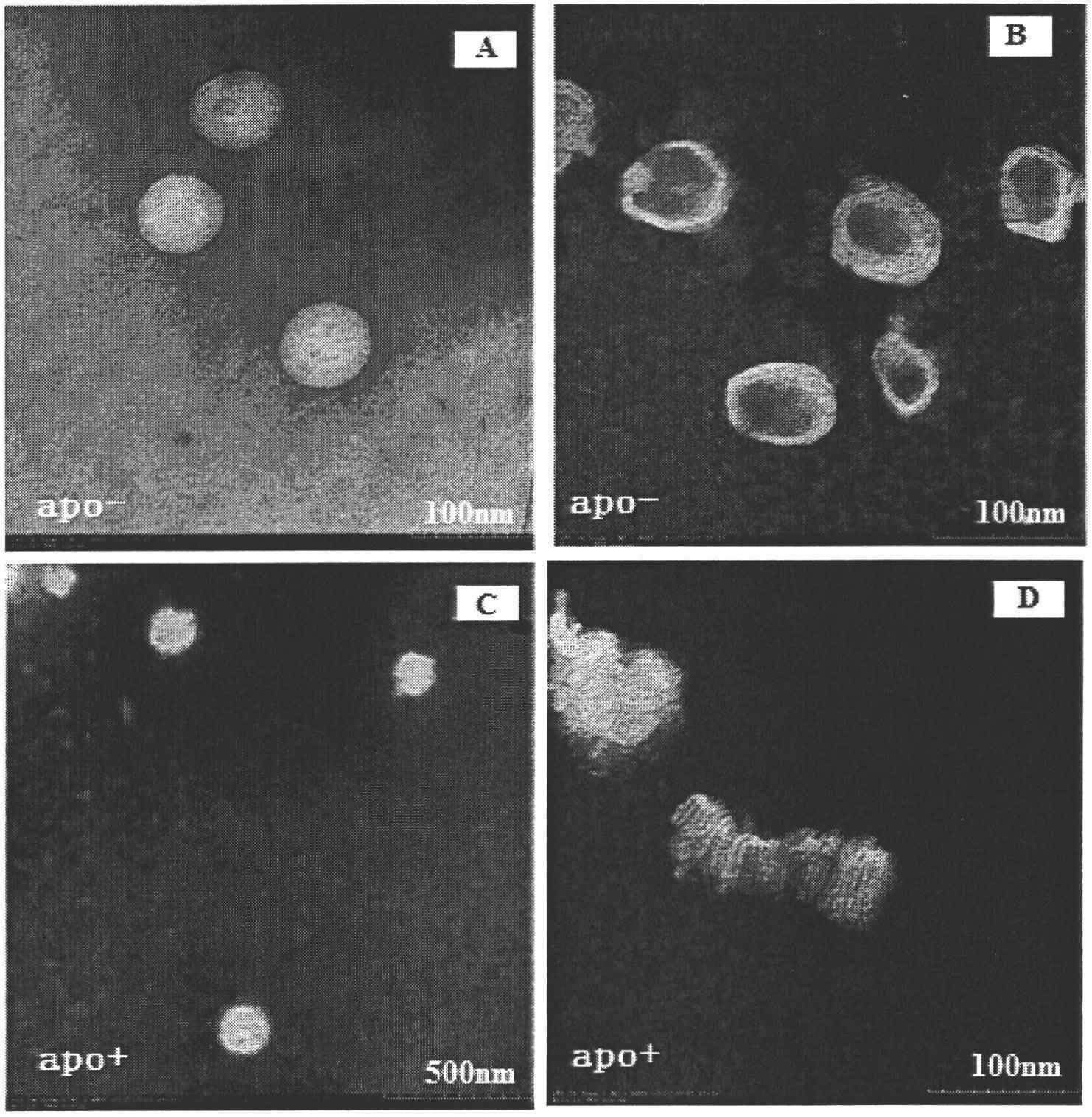
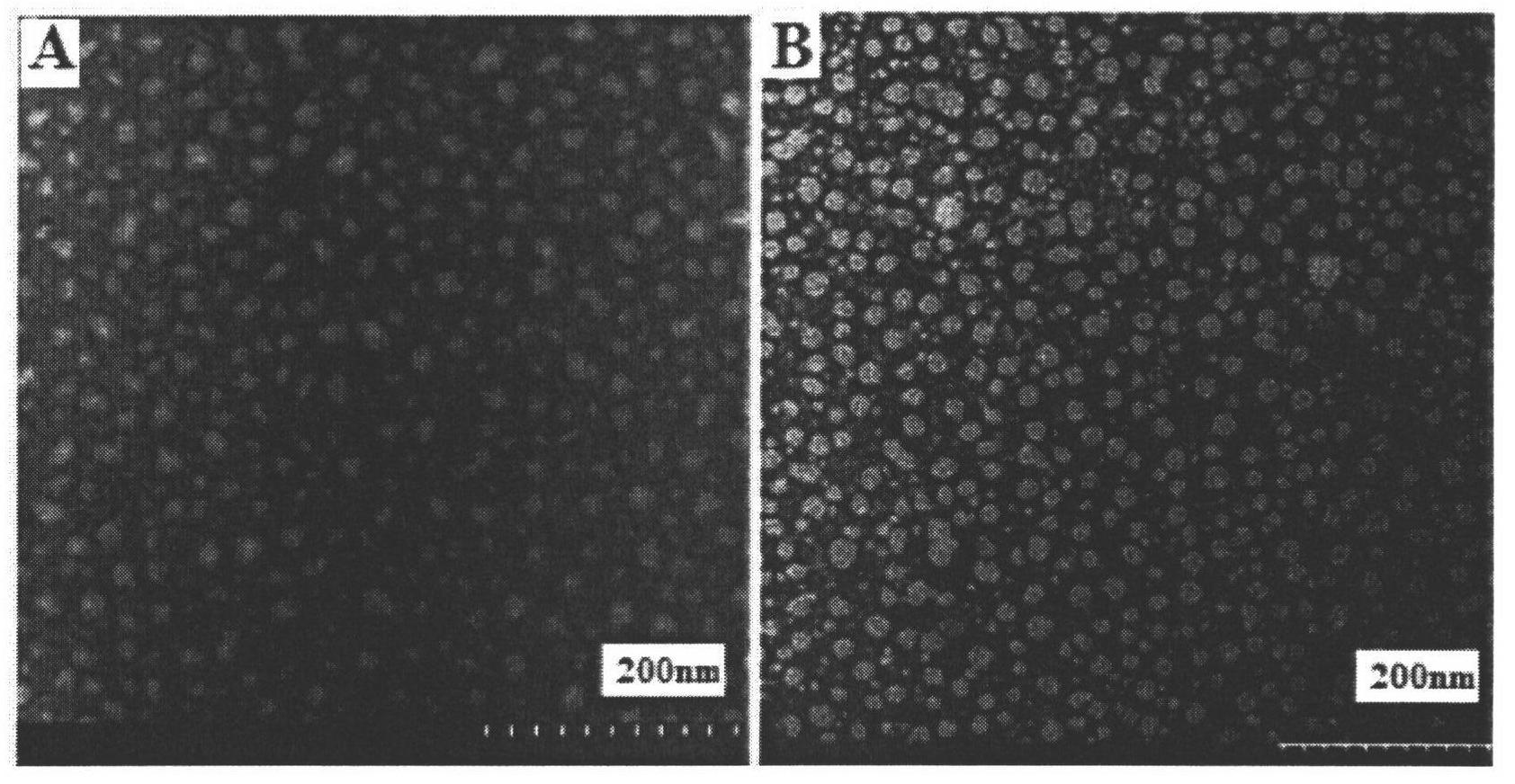
[0035] Adopt transmission electron microscope to observe the form before and after incubation of rHDL and albumen in embodiment 1, see appendix figure 1 In B and D, the liposome...
Embodiment 2
[0037]
[0038] The preparation process is the same as in Example 1. The average particle size of the prepared rHDL after incubation with the protein is 211.5 nm, the encapsulation rate is 89.12%, and the protein binding rate is 44.1%.
[0039] In vitro cell experiment: the mouse cell line RAW264.7 macrophage was used to stimulate the oxidized low-density lipoprotein to replicate the foam cell model, and discoid rHDL was added to the two types of cells to investigate the effect on very low-density lipoprotein (VLDL), Targeting of rHDL under the action of lecithin cholesterol acyltransferase (LCAT), cholesteryl ester transfer protein (CETP), the results showed that the phagocytosis of discoid rHDL in foam cells was 0.19 μg / 1000000 cells, macrophages The uptake of rHDL was 0.084μg / 1000000 cells, there was a significant difference between the two, and the phagocytosis was dependent on VLDL and CETP. After adding LCAT, the phagocytosis of foam cells increased to 0.505μg / 1000000 ...
Embodiment 3
[0042]
[0043] The preparation process is the same as in Example 1. The average particle size of the prepared rHDL after incubation with the protein is 173.4 nm, the encapsulation rate is 88.12%, and the protein binding rate is 61.3%.
[0044] In vitro cell test: as in Example 2, the uptake of disc-shaped rHDL foam cells was 0.21 μg / 1,000,000 cells, and the uptake of macrophages was 0.087 μg / 1,000,000 cells.
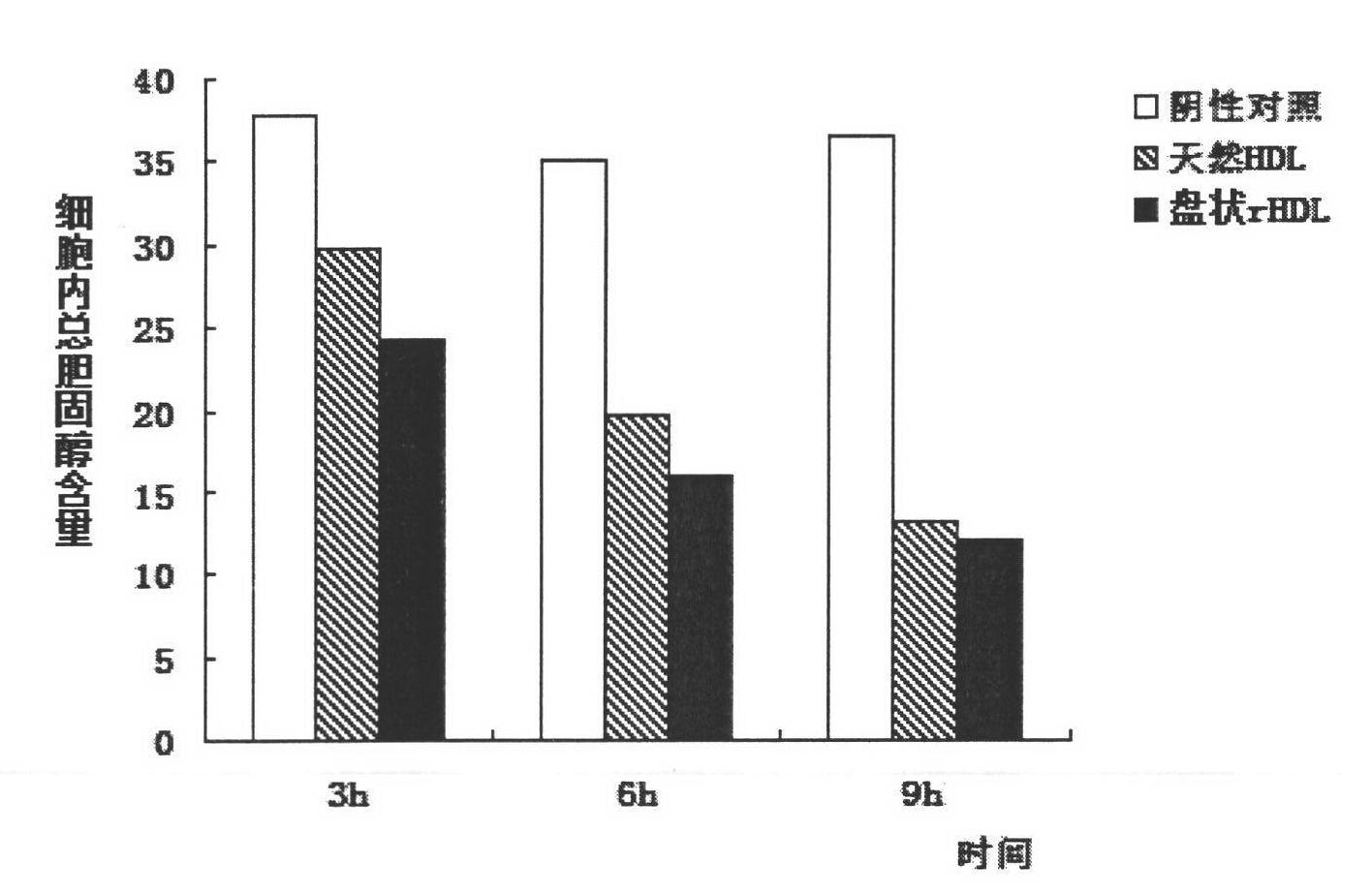
[0045] Cell cholesterol efflux experiment: Same as Example 2, rHDL mediates intracellular cholesterol efflux, and the content is reduced to 41.2% of the initial amount of intracellular cholesterol, indicating that the rHDL constructed with the same components as the natural HDL apolipoprotein is more than containing only apoA-I rHDL has a higher ability to mediate cholesterol efflux.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com